Solution:
Being acidic, it responds with bases to frame salts. It responds with 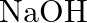 to frame sodium metaborate.
to frame sodium metaborate.
Being acidic, it responds with bases to frame salts. It responds with NaOH to frame sodium silicate
Being acidic, it responds with bases to frame salts. It responds with 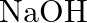 to frame sodium silicate.
to frame sodium silicate.
Being acidic, it responds with bases to frame salts. It responds with 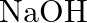 to frame sodium carbonate.
to frame sodium carbonate.
Amphoteric substances respond with the two acids and bases. 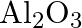 responds with both
responds with both 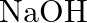 and
and 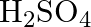
Amphoteric substances respond with the two acids and bases. 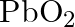 responds with both
responds with both 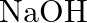 and
and 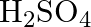
Being essential, it responds with acids to frame salts. It responds with 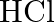 to frame thallium chloride
to frame thallium chloride
