A compound is formed by two elements ![]() and
and ![]() . The element
. The element ![]() forms ccp and atoms of M occupy
forms ccp and atoms of M occupy ![]() of tetrahedral voids. What is the formula of the compound?
of tetrahedral voids. What is the formula of the compound?
A compound forms a hexagonal close-packed structure. What is the total number of voids in 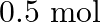 of it? How many of these are tetrahedral voids?
of it? How many of these are tetrahedral voids?
A compound forms a hexagonal close-packed structure. What is the total number of voids in 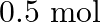 of it? How many of these are tetrahedral voids?
of it? How many of these are tetrahedral voids?
