Ans: The ccp lattice is formed by the atoms of the element ![]() .
.
Here, the number of tetrahedral voids generated is equal to twice the number of atoms of the element ![]() .
.
According to the question, the atoms of element M occupy ![]() of the tetrahedral voids. Therefore, the number of atoms of
of the tetrahedral voids. Therefore, the number of atoms of ![]() is equal to
is equal to ![]() of the number of atoms of
of the number of atoms of ![]() . Therefore, ratio of the number of atoms of
. Therefore, ratio of the number of atoms of ![]() to that of
to that of ![]() is
is ![]() Thus, the formula of the compound is
Thus, the formula of the compound is ![]() .
.
A compound is formed by two elements 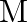 and
and 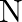 . The element
. The element 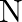 forms ccp and atoms of M occupy
forms ccp and atoms of M occupy 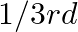 of tetrahedral voids. What is the formula of the compound?
of tetrahedral voids. What is the formula of the compound?
A compound is formed by two elements 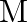 and
and 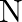 . The element
. The element 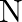 forms ccp and atoms of M occupy
forms ccp and atoms of M occupy 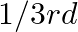 of tetrahedral voids. What is the formula of the compound?
of tetrahedral voids. What is the formula of the compound?
