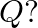Ans: It is given that the atoms of ![]() are present at the corners of the cube. Therefore, number of atoms of
are present at the corners of the cube. Therefore, number of atoms of ![]() in one unit cell
in one unit cell ![]()
It is also given that the atoms of ![]() are present at the body-centre.
are present at the body-centre.
Therefore, number of atoms of ![]() in one unit cell
in one unit cell ![]()
This means that the ratio of the number of ![]() atoms to the number of
atoms to the number of ![]() atoms,
atoms, ![]()
![]()
Hence, the formula of the compound is ![]()
The coordination number of both ![]() and
and ![]() is 8 .
is 8 .
A cubic solid is made of two elements  and
and 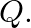 Atoms of
Atoms of 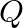 are at the corners of the cube and
are at the corners of the cube and  at the body-centre. What is the formula of the compound? What are the coordination numbers of
at the body-centre. What is the formula of the compound? What are the coordination numbers of  and
and 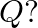
A cubic solid is made of two elements  and
and 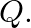 Atoms of
Atoms of 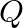 are at the corners of the cube and
are at the corners of the cube and  at the body-centre. What is the formula of the compound? What are the coordination numbers of
at the body-centre. What is the formula of the compound? What are the coordination numbers of  and
and