(i) Hydronium ions (ii) Ammonium ion
(iii) Hydroxyl ion
State the type of bonding present in them.
(b) Give two examples in each case:
(i) Co-ordinate bond compounds
(ii) Solid covalent compounds
(iii) Gaseous polar compounds
(iv) Gaseous non-polar compounds
(v) Liquid non-polar compounds
Answers:
(a)
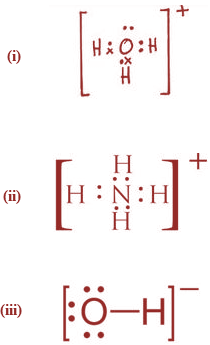
(b)
(i) Co-ordinate bond compounds are Ammonium ion and the hydronium ion.
(ii) Solid covalent compounds are Phosphorus pentachloride and diamond.
(iii) Gaseous polar compounds are Hydrogen chloride and water vapor.
(iv) Gaseous non-polar compounds are Oxygen gas and nitrogen gas.
(v) Liquid non-polar compounds are Toluene and Gasoline.
