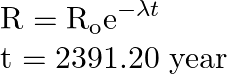![]() activity of a piece of wood from the ruins is given as
activity of a piece of wood from the ruins is given as ![]() per gram
per gram
![]() activity of a living wood is given as
activity of a living wood is given as ![]() per gram
per gram
Half-life of ![]() is given as
is given as ![]() years
years
Using radioactive law, we can write,
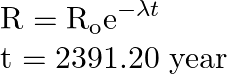
![]() activity of a piece of wood from the ruins is given as
activity of a piece of wood from the ruins is given as ![]() per gram
per gram
![]() activity of a living wood is given as
activity of a living wood is given as ![]() per gram
per gram
Half-life of ![]() is given as
is given as ![]() years
years
Using radioactive law, we can write,