(b) In the given equation –
Identify the role played by conc.H2SO4
1. Non-volatile acid
2. Oxidising agent
3. Dehydrating agent
4. None of the above
(c) Give a balanced equation for Dehydration of concentrated sulphuric acid with sugar crystals
(d) Identify the substance: A dilute mineral acid which forms a white precipitate when treated with a barium chloride solution
Answers:
(a) When Conc.H2SO4 is added to a crystal of hydrated copper sulphate, the water of crystallization is eliminated.
(b) In the given equation, concentrated sulphuric acid acts as an oxidizing agent.
(c) Reaction –
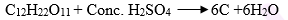
(d) Sulphuric acid is a dilute mineral acid which forms a white precipitate when treated with a barium chloride solution.
