Solution: (2)
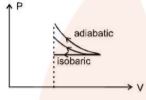
Because the adiabatic process has the largest area under the curve, the amount of work done on the gas is also the largest.
Solution: (2)

Because the adiabatic process has the largest area under the curve, the amount of work done on the gas is also the largest.