Volume of gas is given as ![]() litres
litres ![]()
Gauge pressure is given as ![]() a
a
Temperature is given as ![]()
Universal gas constant is known as ![]()
Let ![]() be the initial number of moles of oxygen gas in the cylinder.
be the initial number of moles of oxygen gas in the cylinder.
We have the gas equation as,
![]()
So,
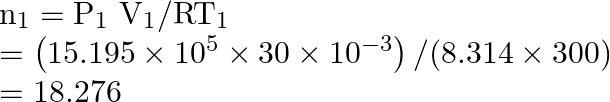
But ![]()
Where,
![]() is the Initial mass of oxygen
is the Initial mass of oxygen
![]() is the Molecular mass of oxygen having value
is the Molecular mass of oxygen having value ![]()
Thus,
![]()
The pressure and temperature drop after some oxygen is removed from the cylinder.
Volume is given as ![]() litres
litres ![]()
Gauge pressure is given as ![]()
![]()
Temperature is given as ![]()
Let the number of moles of oxygen left in the cylinder be ![]() .
.
The gas equation is given as:
![]()
So,
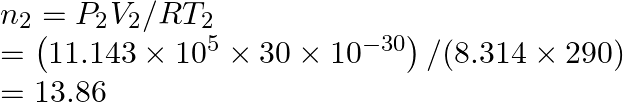
But
![]()
Where,
![]() mass of oxygen remaining in the cylinder
mass of oxygen remaining in the cylinder
Hence,
![]()
The mass of oxygen taken out of the cylinder can be calculated using the following formula: Mass of oxygen in the cylinder at the start – Mass of oxygen in the cylinder at the end
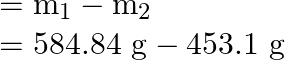
We get,
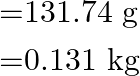
As a result, mass of oxygen is taken out of the cylinder is ![]() .
.
