(i) Write the electron-dot structures for sodium and oxygen.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii)What are the ions present in these compounds?
Answer:
(i) Sodium

Oxygen

(ii) Magnesium oxide formation:
When magnesium combines with oxygen, its two outermost electrons are transferred to an oxygen atom. The magnesium atoms produce a magnesium ion (Mg2+) by losing two electrons, and the oxygen atom makes an oxide ion by acquiring two electrons (O2-).
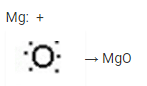
Sodium oxide formation:
The two outermost electrons of two sodium atoms are transferred to an oxygen atom. The two sodium atoms generate sodium ions (2Na+) by losing two electrons. The oxygen atom gains two electrons and produces an oxide ion (O2-).
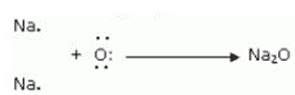
(iii) Sodium ions (2Na+) and oxide ions are found in sodium oxide complex (Na2O) (O2-).
Magnesium ions Mg2+ and oxide ions are present in Magnesium oxide complex (MgO) (O2-).
