Answer:
(a) The liquid and vapor phases of 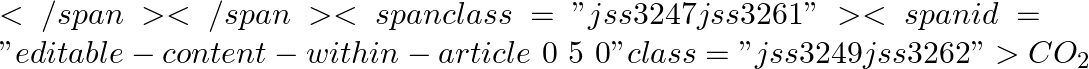 coexist at the triple point temperature and pressure of − 56.6 degrees Celsius and 5.11 atmospheres.
coexist at the triple point temperature and pressure of − 56.6 degrees Celsius and 5.11 atmospheres.
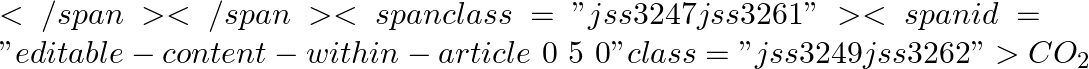 coexist at the triple point temperature and pressure of − 56.6 degrees Celsius and 5.11 atmospheres.
coexist at the triple point temperature and pressure of − 56.6 degrees Celsius and 5.11 atmospheres.
(b) When the pressure of 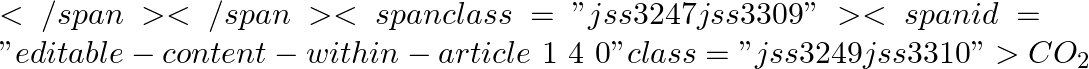 is reduced, both the boiling point and the freezing point of
is reduced, both the boiling point and the freezing point of 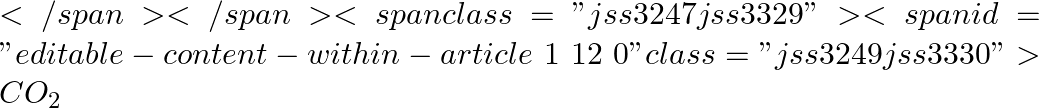 decrease.
decrease.
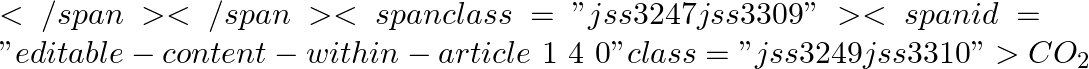 is reduced, both the boiling point and the freezing point of
is reduced, both the boiling point and the freezing point of 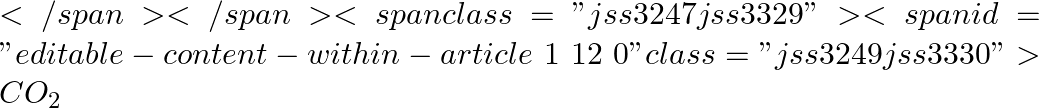 decrease.
decrease.