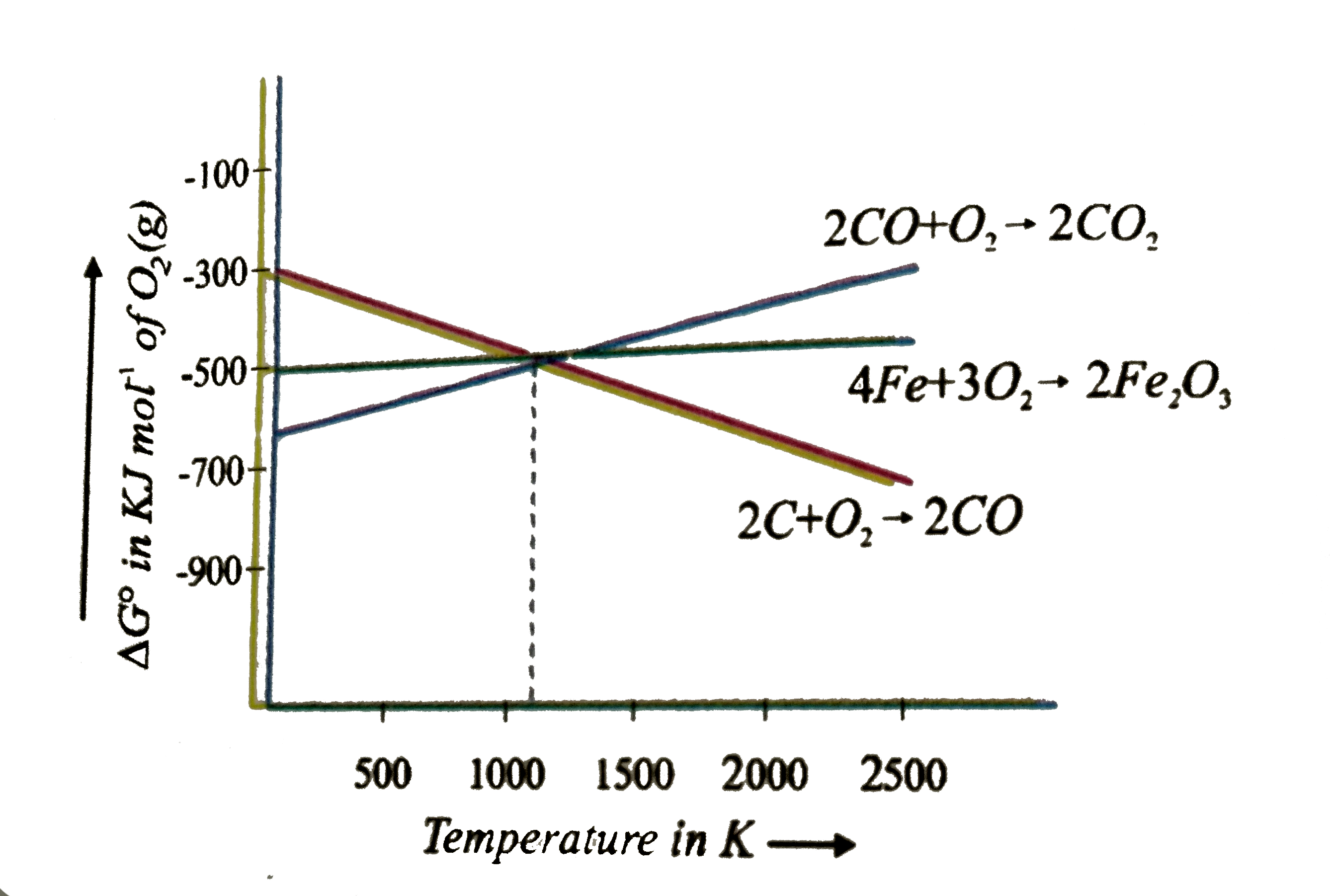
The ∆G formation of FeO is a bit negative than the ∆G formation of carbon monoxide from carbon. Summation of both ∆G will be negative at about 1073K. Above 1073K the FeO production line exceeds the oxidation line of C to CO. Therefore, at this stage, coke will reduce to FeO and will also be oxidize to CO.
