Answer: Because Magnesium metal combines with ambient oxygen to generate Magnesium Oxide (MgO) layer, which is a very stable chemical, it is recommended that magnesium ribbon be cleaned prior to...
A doctor has prescribed a corrective lens of power +1.5 D. Find the focal length of the lens. Is the prescribed lens diverging or converging?
Answer- Expression for power of lens (P) = 1/f We are given that P = 1.5D Therefore, f = 1/1.5 f = 10/15 So, f = 0.66 m
Find the focal length of a lens of power -2.0 D. What type of lens is this?
Answer- According to the question, Power of the lens (P) is P = -2D And the expression for the power of the lens is P = 1/f So, we get => f = -1/2 f = -0.5 m As it has a negative focal length....
An object 5 cm is placed at a distance of 20 cm in front of a convex mirror of radius of curvature 30 cm. Find the position, nature and size of the image.
Answer- According to the question, object distance (u) is – 20 cm And object height (h) is 5 cm Radius of curvature (R) is 30 cm And we know that radius of curvature = 2 × Focal length Or, R = 2f We...
The magnification produced by a plane mirror is +1. What does this mean?
Answer- A virtual and erect image generated by a flat mirror is denoted by a positive sign. The size of the image is equal to the size of the object because the magnification is 1.
An object is placed at a distance of 10 cm from a convex mirror of focal length 15 cm. Find the position and nature of the image.
Answer- According to the focal length of convex mirror, (f) is +15 cm And the object distance (u) is – 10 cm Using the mirror formula, 1/f = 1/v - 1/u Or, 1/v = 1/f + 1/u = 1/15 - 1/(-10) v = 5/30 =...
A concave lens of focal length 15 cm forms an image 10 cm from the lens. How far is the object placed from the lens? Draw the ray diagram.
Answer- According to the question, focal length of concave lens (OF1) is f = – 15 cm Image distance (v) is – 10 cm Using the lens formula - 1/f = 1/v - 1/u Or, 1/v = 1/f + 1/u = - 1/(10) - 1/(-15)...
An object 5 cm in length is held 25 cm away from a converging lens of focal length 10 cm. Draw the ray diagram and find the position, size and the nature of the image formed.
Answer- According to the question, height of the Object h0 is 5 cm And the distance of the object from converging lens (u) is -25 cm Also the Focal length of a converging lens is f = 10 cm...
One-half of a convex lens is covered with a black paper. Will this lens produce a complete image of the object? Verify your answer experimentally. Explain your observations.
Answer- Yes, as indicated in the illustration, it will provide a comprehensive image of the object. The image of a distant object, such as a tree. on a screen when the lower part of the lens is...
Name the type of mirror used in the following situations.
(a) Headlights of a car (b) Side/rear-view mirror of a vehicle (c) Solar furnace Support your answer with reason. Answer- (a) Concave Because when the light source is placed at their major focus,...
We wish to obtain an erect image of an object, using a concave mirror of focal length 15 cm. What should be the range of distance of the object from the mirror? What is the nature of the image? Is the image larger or smaller than the object? Draw a ray diagram to show the image formation in this case.
Answer- The object's distance from the mirror's pole ranges from 0 to 15 cm. The image's nature is virtual, upright, and larger than the thing.
Which of the following lenses would you prefer to use while reading small letters found in a dictionary?
(a) A convex lens of focal length 50 cm (b) A concave lens of focal length 50 cm (c) A convex lens of focal length 5 cm (d) A concave lens of focal length 5 cm Answer – The correct option is (c)....
A spherical mirror and a thin spherical lens have a focal length of -15 cm. The mirror and the lens are likely to be
(a) both concave (b) both convex (c) the mirror is concave and the lens is convex (d) the mirror is convex, but the lens is concave Answer – (a) Both concave The focal length of a concave mirror and...
Where should an object be placed in front of a convex lens to get a real image of the size of the object?
(a) At the principal focus of the lens (b) At twice the focal length (c) At infinity (d) Between the optical centre of the lens and its principal focus. Answer – The correct option is (b). The...
The image formed by a concave mirror is observed to be virtual, erect and larger than the object. Where should be the position of the object?
(a) Between the principal focus and the centre of curvature (b) At the centre of curvature (c) Beyond the centre of curvature (d) Between the pole of the mirror and its principal focus. Answer- The...
Which one of the following materials cannot be used to make a lens?
(a) Water (b) Glass (c) Plastic (d) Clay Answer – The correct option is (d). Clay cannot be used to create a lens because light rays cannot flow through it.
Find the power of a concave lens of focal length 2 m.
Answer- According to the question, focal length of concave lens (f) is 2 m Power of lens (P) = 1/f Power = 1/ (-2) Therefore, Power = -0.5D
A convex lens forms a real and inverted image of a needle at a distance of 50 cm from it. Where is the needle placed in front of the convex lens if the image is equal to the size of the object? Also, find the power of the lens.
Answer- Because the image is actual and the same size, it should be positioned at 2F. The image of the needle is assumed to be formed at a distance of 50 cm from the convex lens. As a result, the...
Define 1 dioptre of power of a lens.
Answer- The symbol D stands for dioptre, which is the SI unit of lens power. A dioptre is the power of a lens with a focal length of one metre.
The refractive index of diamond is 2.42. What is the meaning of this statement?
Answer- Because diamond has a refractive index of 2.42, the speed of light in it is reduced by a factor of 2.42 when compared to the speed of light in air. To put it another way, the speed of light...
You are given kerosene, turpentine and water. In which of these does the light travel fastest? Use the information given in Table.
Material mediumRefractive indexMaterial mediumRefractiveindexAir1.0003Canada Balsam1.53Ice1.31––Water1.33Rock salt1.54Alcohol1.36––Kerosene1.44Carbon disulphide1.63Fusedquartz1.46Denseflint...
Find out, from Table, the medium having highest optical density. Also find the medium with lowest optical density.
MaterialmediumRefractive indexMaterial mediumRefractiveindexAir1.0003Canada Balsam1.53Ice1.31––Water1.33Rock salt1.54Alcohol1.36––Kerosene1.44Carbon disulphide1.63Fusedquartz1.46Denseflint...
Light enters from air to glass having refractive index 1.50. What is the speed of light in the glass? The speed of light in vacuum is 3 x 108 ms-1.
Answer- According to the question, speed of light in vacuum (c) is 3 × 108 m/s And refractive index of glass (ng) is 1.50 Expression for the speed of light in the glass (v) is given by - v = Speed...
A ray of light travelling in air enters obliquely into water. Does the light ray bends towards the normal or away from the normal? Why?
Answer- The light ray bends in the direction of normal. When a light ray passes through an optically rarer medium (low refractive index) and enters an optically denser medium (high refractive...
A concave mirror produces three times magnified (enlarged) real image of an object placed at 10 cm in front of it. Where is the image located?
Answer- Expression for magnification produced by a spherical mirror is - m = (height of image)/(height of object) Also, m = -(Image distance)/(object distance) So, we can write - (height of...
The radius of curvature of a spherical mirror is 32 cm. What is its focal length?
Answer- According to the question, radius of curvature (R) is 32 cm Expression for radius of curvature of the spherical mirror = 2 × Focal length (f) R = 2f Or, f= R/2 f= 32 / 2 f = 16 Therefore, 16...
Why do we prefer a convex mirror as a rear-view mirror in vehicles?
Answer- In cars and vehicles, a convex mirror is favoured as a rear-view mirror. This is because it provides a broader field of view, allowing the driver to see more of the traffic behind him....
Name the mirror that can give an erect and enlarged image of an object.
Answer- Concave Mirror is a mirror that may magnify and construct an object's image.
The radius of curvature of a spherical mirror is 20 cm. What is its focal length?
Answer- According to the question, radius of curvature (R) is 20 cm Expression for radius of curvature of the spherical mirror is = 2 × Focal length (f) So, R = 2f Or, f= R/2 = 20 / 2 f = 10...
Define the principal focus of a concave mirror.
Answer- After reflecting from a concave mirror, light beams that are parallel to the principal axis converge at a certain location on the principal axis. The spot is the principal focus of the...
In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
(a) Lead sulphate (insoluble)
(b) Lead acetate
(c) Ammonium nitrate
(d) Potassium sulphate
Solution: The correct response is (b) lead acetate. Explanation: Because we need a compound that contains lead in order to obtain lead iodide, ammonium nitrate and potassium sulphate are ruled out....
Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is
(a) 1:1
(b) 2:1
(c) 4:1
(d) 1:2
Answer: option b Water contains two moles of hydrogen and one mole of water in a single mole. Because of this, the mole ratio of hydrogen to oxygen is 2:1.
Barium chloride on reacting with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved?
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction
(a) (i) only
(b) (ii) only
(c) (iv) only
(d) (ii) and (iv)
Solution: Answer is option d) The elements ammonium and barium are being displaced from their respective salts, according to the data. As a result, we have a double displacement reaction. Because of...
Which among the following statement(s) is (are) true? Exposure of silver chloride to sunlight for a long duration turns grey due to
(i) the formation of silver by decomposition of silver chloride
(ii) sublimation of silver chloride
(iii) decomposition of chlorine gas from silver chloride
(iv) oxidation of silver chloride
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (iv) only
Solution: Answer is option a) Silver halides, particularly silver chloride, decompose in the presence of sunlight, resulting in the formation of silver metal and a halogen gas (silver) (chlorine or...
Solid calcium oxide reacts vigorously with water to form calcium hydroxide accompanied by the liberation of heat. This process is called slaking of lime. Calcium hydroxide dissolves in water to form its solution called lime water. Which among the following is (are) true about slaking of lime and the solution formed?
(i) It is an endothermic reaction
(ii) It is an exothermic reaction
(iii) The pH of the resulting solution will be more than seven
(iv) The pH of the resulting solution will be less than seven
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (iii) and (iv)
Solution: The answer is (b) (ii) and (iii) In the presence of vigorous water reaction, solid calcium oxide transforms into calcium hydroxide, which is accompanied by the production of heat. This...
Which among the following is(are) double displacement reaction(s)?
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2 SO4 + BaCl2 → BaSO4 + 2NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2O2 → CO2 + 2H2O
(a) (i) and (iv)
(b) (ii) only
(c) (i) and (ii)
(d) (iii) and (iv)
Solution: The answer is (b) (ii) only Sodium and barium are both displaced from their respective salts in this reaction, which is referred to as a double displacement reaction.
A dilute ferrous sulphate solution was gradually added to the beaker containing acidified permanganate solution. The light purple colour of the solution fades and finally disappears. Which of the following is the correct explanation for the observation?
(a) KMnO4 is an oxidising agent, it oxidises FeSO4
(b) FeSO4 acts as an oxidising agent and oxidises KMnO4
(c) The colour disappears due to dilution; no reaction is involved
(d) KMnO4 is an unstable compound and decomposes in the presence of FeSO4 to a colourless compound.
Solution: Answer is (a) KMnO4 is an oxidising agent, it oxidises FeSO4 Potassium permanganate (KMnO4) is used as an oxidising agent during this reaction. This is due to the presence of KMnO4, which...
Three beakers labelled as A, B and C each containing 25 mL of water were taken. A small amount of NaOH, anhydrous CuSO4 and NaCl were added to the beakers A, B and C respectively. It was observed that there was an increase in the temperature of the solutions contained in beakers A and B, whereas, in the case of beaker C, the temperature of the solution falls. Which one of the following statements(s) is(are) correct?
(i) In beakers A and B, the exothermic process has occurred.
(ii) In beakers A and B, the endothermic process has occurred.
(iii) In beaker C exothermic process has occurred.
(iv) In beaker C endothermic process has occurred.
(a) (i) only
(b) (ii) only
(c) (i) and (iv)
(d) (ii) and (iii)
Answer: Answer is (c) (i) and (iv) Exothermic processes result in the release of enormous amounts of heat – as a result, when water reacts with quick lime and when acid reacts with water, heat...
Which of the following are exothermic processes?
(i) The reaction of water with quick lime
(ii) Dilution of an acid
(iii) Evaporation of water
(iv) Sublimation of camphor (crystals)
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (iii) and (iv)
Answer: Option a) Exothermic processes result in the release of enormous amounts of heat – as a result when water reacts with quick lime and when an acid reacts with water, heat energy is...
Which of the following statements about the given reaction are correct? 3Fe(s) + 4H2O(g) → Fe3O4 (s) + 4H2 (g)
1. Iron metal is getting oxidised
2. Water is getting reduced
3. Water is acting as a reducing agent
4. Water is acting as an oxidising agent
(a) (i), (ii) and (iii)
(b) (iii) and (iv)
(c) (i), (ii) and (iv)
(d) (ii) and (iv)
Answer: Option c) In this reaction, oxygen reacts with water to form oxidised water. Because oxygen is being removed from water, the water's oxygen content is being reduced. Water is a source of...
The following reaction is an example of a:4NH3 (g) + 5O2 (g) → 4NO(g) + 6H2O(g)
(i) displacement reaction
(ii) combination reaction
(iii) redox reaction
(iv) neutralisation reaction
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (iii) and (iv)
Solution: Answer is (c) (i) and (iii) The reaction described here is a combination of displacement and redox reactions. The displacement reaction occurs when oxygen displaces hydrogen in ammonia,...
Which of the following is not a physical change?
(a) Boiling of water to give water vapour
(b) Melting of ice to give water
(c) Dissolution of salt in water
(d) Combustion of Liquefied Petroleum Gas (LPG)
Answer: Answer is (d) Combustion of Liquefied Petroleum Gas (LPG) Because a new compound is formed after burning, combustion is always accompanied by a chemical change, which is irreversible in...
Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(c) Ethanol is warmed with ethanoic acid to form ethyl acetate in the presence of concentrated 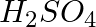
(d) Ethene is burnt in the presence of oxygen to form carbon dioxide, water and releases heat and light.
Answer: (c) $C_2 H_5 OH ( aq )+ CH_3 COOH (l) \longrightarrow CH_3 COO C_2 H_5( aq )+ H_2 O ( t )$It is a neutralisation reaction and double displacement reaction.(d) $C_{2} H_{4}( g )+3 O_{2}(g)...
Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(a) Nitrogen gas is treated with hydrogen gas in the presence of a catalyst at 773K to form ammonia gas.
(b) Sodium hydroxide solution is treated with acetic acid to form sodium acetate and water.
Answer: (a) $N {2}( g )+3 H {2}( g )=\frac{\text { catalyst }}{773 K } 2 NH {2}( g )$ It is a combination reaction. (b) $NaOH ( aq )+ CH _{3} COOH ( aq ) \longrightarrow CH_3 COONa ( aq )+ H_2 O...
Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(c) Chlorine gas is passed in an aqueous potassium iodide solution to form potassium chloride solution and solid iodine.
(d) Ethanol is burnt in air to form carbon dioxide, water and releases heat.
Answer: c) Aqueous potassium iodide solution is passed through a chlorine gas stream, resulting in the formation of potassium chloride solution and solid iodine.This is a reaction with a single...
Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(a) Thermite reaction, iron (III) oxide reacts with aluminium and gives molten iron and aluminium oxide.
(b) Magnesium ribbon is burnt in an atmosphere of nitrogen gas to form solid magnesium nitride.
Answer: a) Iron (III) oxide reacts with aluminium to form molten iron and aluminium oxide in the first step of the Thermit reaction. This is a reaction with a single displacement. Fe2O3 + 2Al →...
Complete the missing components/variables given as x and y in the following reactions
(c) 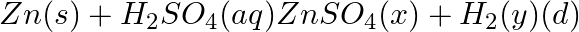 CaCO_3 (s) →xCaO(s) + CO_2 (g)$
CaCO_3 (s) →xCaO(s) + CO_2 (g)$
Solution: (c) Zn(s) + H2 SO4 (aq) → ZnSO4 (aq) + H2 (g) (d) CaCO3 (s) →heat→ CaO(s) + CO2 (g) x-(aq) y(g)x is heat
Complete the missing components/variables given as x and y in the following reactions
(a) 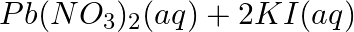 →
→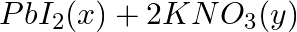
(b) 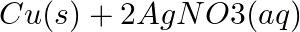 →
→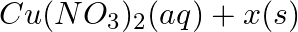
Answer: (a) Pb(NO3 ) 2 (aq) + 2KI(aq) → PbI2 (s) + 2KNO3 (aq) (b) Cu(s) + 2Ag NO3 (aq) → Cu(NO3 ) 2 (aq) + 2Ag(s) x(s), y (aq)x is 2Ag
Which among the following changes are exothermic or endothermic in nature?
(a) Decomposition of ferrous sulfate
(b) Dilution of sulphuric acid
(d) Dissolution of ammonium chloride in water
Answer:An exothermic process is one that generates heat as a byproduct. A portion of this heat is transferred to the surrounding environment. Heat must be supplied to the system from the surrounding...
Identify the reducing agent in the following reactions
(a) 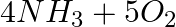

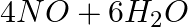
(b) 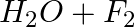

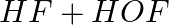
Answer: In a redox chemical reaction, a reducing agent is an element or compound that "donates" or "loses" an electron to an electron recipient as part of the reaction. NH3-AmmoniaH2O – Water
Identify the reducing agent in the following reactions
(c) 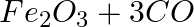 →
→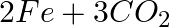
(d) 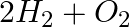 →
→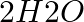
Answer: In a redox chemical reaction, a reducing agent is an element or compound that "donates" or "loses" an electron to an electron recipient as part of the reaction. CO-Carbon momnoxide$H_2$-...
Identify the oxidizing agent (oxidant) in the following reactions
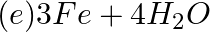 →
→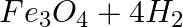
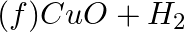 →
→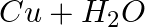
Answer: According to chemistry, an oxidising agent, or oxidising agent, is a substance that has the ability to oxidise other substances, or to accept electrons from them, in other words, it is an...
Identify the oxidizing agent (oxidant) in the following reactions
(c) 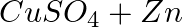 →
→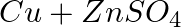
(d) 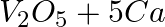 →
→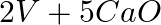
Answer:According to chemistry, an oxidising agent, or oxidising agent, is a substance that has the ability to oxidise other substances, or to accept electrons from them, in other words, it is an...
Identify the oxidizing agent (oxidant) in the following reactions
(a) 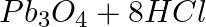 →
→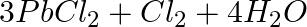
(b) 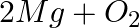 →
→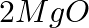
Answer:According to chemistry, an oxidising agent, or oxidising agent, is a substance that has the ability to oxidise other substances, or to accept electrons from them, in other words, it is an...
Write the balanced chemical equations for the following reactions (c) Copper sulfate on treatment with potassium iodide precipitates cuprous iodide (Cu2 I2 ), liberates iodine gas, and also forms potassium sulfate.
Solution: (c) 2CuSO4+4Kl →$2K_2SO_4+Cu_2I_2+I_2$
Write the balanced chemical equations for the following reactions
(a) Sodium carbonate on reaction with hydrochloric acid in equal molar concentrations gives sodium chloride and sodium hydrogen carbonate.
(b) Sodium hydrogen carbonate on reaction with hydrochloric acid gives sodium chloride, water and liberates carbon dioxide.
Answer: (a) Na2CO3 + HCl → NaCl + NaHCO3 (b) NaHCO3 + HCl → NaCl + H2O + CO2
In a solution of potassium chloride when mixed with silver nitrate solution, an insoluble white substance is formed. Write the chemical reaction involved and also mention the type of chemical reaction?
Solution: Double displacement reactions are chemical reactions in which one component of each of the reacting molecules is exchanged in order to form the products, as defined by the American...
Ferrous sulfate decomposes with the evolution of a gas having a characteristic odor of burning sulfur. Write the chemical reaction involved and identify the type of reaction.
Answer: When heated, ferrous sulfate decomposes into ferric oxide, sulphur dioxide, and sulphur trioxide, which are all toxic. Using this balanced equation, we can express the thermal decomposition...
Why do fireflies glow at night?
Answer: During the course of the night, fireflies produce a chemical reaction within their bodies that allows them to glow. In the presence of an enzyme known as luciferase, oxygen reacts with...
Grapes hanging on the plant do not ferment but after being plucked from the plant can be fermented. Under what conditions do these grapes ferment? Is it a chemical or a physical change?
Answer: The defense mechanism of plants prevents the fermentation of grapes on the plant. When grapes are plucked from the vine, they react with yeast to produce fermentation, which is the final...
During the reaction of some metals with dilute hydrochloric acid, the following observations were made.
(a) Silver metal does not show any change
(b) The temperature of the reaction mixture rises when aluminum (Al) is added.
Answer: a) Because silver is a member of the low reactive series of metals, there will be no reaction between it and dilute hydrogen chloride. b) Due to the fact that it is an exothermic reaction,...
During the reaction of some metals with dilute hydrochloric acid, the following observations were made.
(c) The reaction of sodium metal is found to be highly explosive
d) Some bubbles of gas are seen when lead (Pb) is reacted with the acid.
Answer: c) Sodium is a highly reactive metal, and when it reacts with atmospheric oxygen, it produces an exothermic reaction, which raises the temperature of the surrounding environment. d) Hydrogen...
A substance X, which is an oxide of a group 2 element, is used intensively in the cement industry. This element is present in bones also. On treatment with water, it forms a solution that turns red litmus blue. Identify X and also write the chemical reactions involved.
Answer: Calcium oxide is the chemical compound X. CaO is a compound that is widely used in the cement industry. Water treatment of CaO results in the formation of Ca(OH)2, which is alkaline in...
Write a balanced chemical equation for each of the following reactions and also classify them.
(c) Iron (III) oxide on heating with carbon monoxide gas reacts to form solid iron and liberates carbon dioxide gas.
(d) Hydrogen sulfide gas reacts with oxygen gas to form solid sulfur and liquid water.
Answer: (c) Fe2O3 + 3CO + 2Fe + 3CO2 This is a redox reaction. (d) 2 H2S+O2 → 2 s + 2 H2O This is a replacement reaction.
Write a balanced chemical equation for each of the following reactions and also classify them.
(a) Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic acid solution.
(b) A piece of sodium metal is added to absolute ethanol to form sodium ethoxide and hydrogen gas.
Answer: (a) Pb(CH3COO)2 + 2HCI – PbCl2 + CH3COOH This is a Double Displacement reaction. (b) 2Na + 2C2H5OH + 2C2H5ONa+ H2 This is a Displacement reaction.
Why do we store silver chloride in dark coloured bottles?
Answer: When exposed to sunlight, silver chloride decomposes into silver and chlorine gas, which are both toxic. As a result, silver chloride is stored in bottles that are dark in colour.
Balance the following chemical equations and identify the type of chemical reaction.
(c) Na(s) + S(s) → Fuse Na2S(s)
(d) TiCl4 (l) + Mg(s) → Ti(s) + MgCl2 (s)
Answer: (c) 2Na(s) + S(s) — (Fuse) → Na2S(s) This is an example of a reaction known as a Combination reaction. (d) TiCI4 (1) + Mg(s) → Ti(s) + 2MgCl2 (s) There are several types of displacement...
Balance the following chemical equations and identify the type of chemical reaction.
(e) CaO(s) + SiO2 (s) → CaSiO3 (s)
(f) H2O2 (l) → U V H2O(l) + O2 (g)
Solution: (e) Cao(s) + SIO2(s) + CaSIO3(s) This reaction falls under the category of Displacement reactions (f) 2H2O2 (I) — UV → 2H2O(I) + O2 (g) This is a decomposition reaction.
Balance the following chemical equations and identify the type of chemical reaction.
(a) Mg(s) + 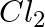 (g) →
(g) → 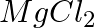 (s)
(s)
(b) HgO(s) → Heat Hg(l) + 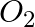 (g)
(g)
Answer: (a) Mg(s) + Cl2(g) → MgCl2(s) A combination reaction, also known as a synthesis reaction, is a type of reaction in which two or more substances are combined. (b) 2HgO(s) — (Heat) → 2 Hg(I) +...
A magnesium ribbon is burnt in oxygen to give a white compound X accompanied by the emission of light. If the burning ribbon is now placed in an atmosphere of nitrogen, it continues to burn and forms a compound Y.
(a) Write the chemical formulae of X and Y.
(b) Write a balanced chemical equation, when X is dissolved in water.
Solution: a) It is formed when magnesium ribbon is burned in oxygen with the emission of light and heat energy that magnesium oxide is formed.X is a compound with the chemical formula MgO. After...
Zinc liberates hydrogen gas when reacted with dilute hydrochloric acid, whereas copper does not. Explain why?
Answer: Copper is more reactive than zinc because zinc is placed above hydrogen in the activity series of metals, whereas copper is placed below hydrogen in the activity series of metals. As a...
A silver article generally turns black when kept in the open for a few days. The article when rubbed with toothpaste again starts shining. (a) Why do silver articles turn black when kept in the open for a few days? Name the phenomenon involved. (b) Name the black substance formed and give its chemical formula.
Solution: A reaction between silver and H2S in the atmosphere results in the formation of Silver Sulphide, which is a dark brown compound with a sulfuric acid smell. Corrosion is the term used to...
On heating blue colored powder of copper (II) nitrate in a boiling tube, copper oxide (black), oxygen gas and a brown gas X are formed
(c) Identify the type of reaction.
(d) What could be the pH range of the aqueous solution of the gas X?
Answer: c) Thermal decomposition is the reaction that is taking place. d) Due to the fact that NO2 dissolves in water and forms an acidic solution, pH is less than 7. (pH range below 7).
On heating blue coloured powder of copper (II) nitrate in a boiling tube, copper oxide (black), oxygen gas and a brown gas X is formed
(a) Write a balanced chemical equation of the reaction.
(b) Identity the brown gas X evolved.
Answer: a) b) Nitrogen dioxide, also known as $NO_2$ , is the brown gas X.
Give the characteristic tests for the following gases
a) 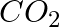
b) 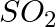
Answer: a) Pass CO2 through limewater, which will turn the water milky in colour. A confirmation test for the presence of Carbon-di-oxide is carried out here. b) The smell of SO2 is one of its...
Give the characteristic tests for the following gases
c) 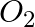 d)
d)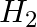
Answer: c) The oxygen test involves lighting a match stick in the presence of oxygen, which causes the match stick to burn even more brightly. d) Burning matchstick is brought close to H2 gas and...
What happens when a piece of (c) silver metal is added to copper sulphate solution? Write a balanced equation.
Answer: c)There will be no reaction when silver metal is added to Copper Sulphate solution because silver is a non-reactive metal.
What happens when a piece of
(a) zinc metal is added to copper sulfate solution?
(b) aluminum metal is added to dilute hydrochloric acid? Write a balanced equation.
Solution: A) When zinc is added to a solution of copper sulfate, zinc displaces copper and forms zinc sulphate as a result. Zn(s)+ CuSo4(aq)→ ZnSo4(aq)+ Cu(s) b) When aluminium metals react with...
What happens when zinc granules are treated with a dilute solution of 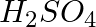 , HCl,
, HCl, 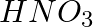 , NaCl and NaOH, also write the chemical equations if a reaction occurs.
, NaCl and NaOH, also write the chemical equations if a reaction occurs.
Solution: Displacement reaction occurs when zinc granules react with concentrated H2SO4, resulting in the formation of ZnSO4 and the release of hydrogen gas. Zn( s)+ H2SO4(aq)→ ZnSo4 (aq)+H2 (g)...
On adding a drop of barium chloride solution to an aqueous solution of sodium sulfite, a white precipitate is obtained.
(c) On adding dilute hydrochloric acid to the reaction mixture, white precipitate disappears. Why?
Solution: c) When we add dilute $HCl$ to this reaction mixture, we get the following products: barium chloride, sulphur dioxide, and water. Due to the fact that barium chloride is a soluble...
On adding a drop of barium chloride solution to an aqueous solution of sodium sulfite, a white precipitate is obtained.
(a) Write a balanced chemical equation of the reaction involved
(b) What other name can be given to this precipitation reaction?
Solution: a)The reaction of a drop of Barium Chloride solution with one drop of sodium sulphite solution results in the formation of barium sulphite, a white precipitate of barium sulphite....
You are provided with two containers made up of copper and aluminum. You is also provided with solutions of dilute HCl, dilute 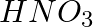 ,
, 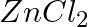 and
and 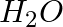 . In which of the above containers these solutions can be kept?
. In which of the above containers these solutions can be kept?
Answer: Due to the fact that copper is a noble metal and will not react with HCl or even $HNO_3$, it is possible to store this solution in a copper container. Keeping the solution in an aluminium...
Which of the following ray diagrams is correct for the ray of light incident on a lens shown in the figure?
(a) Fig. A. (b) Fig. B. (c) Fig. C. (d) Fig. D. Answer: (a) Fig. A. Explanation: The ray diagram in figure A is correct for the light ray incident on a lens as the incident ray travels through the...
The path of a ray of light coming from air passing through a rectangular glass slab traced by four students are shown as A, B, C and D in the Figure. Which one of them is correct?
(a) A (b) B (c) C (d) D Answer: b) B Explanation: The path of a ray of light coming from air passing through a rectangular glass slab traced by the student B is correct.
A beam of light is incident through the holes on side A and emerges out of the holes on the other face of the box as shown in the figure. Which of the following could be inside the box?
(a) Concave lens (b) Rectangular glass slab (c) Prism (d) Convex lens Answer: (d) Convex lens Explanation: When a beam of light is incident through the holes on side A and emerges out of the holes...
Find out the following in the electric circuit given in Figure 12.9
(c) Potential difference across 4 Ω resistance
(d) Power dissipated in 4 Ω resistor (e) Difference in ammeter readings, if any
Solution: The potential difference across $4 \Omega$ resistor is potential drop by the $4 \Omega$ resistor.$V=I R=1 \times 4=4 V$The power dissipated in $4 \Omega$ resistor$$P=I^{2} R=1^{2} \times...
Find out the following in the electric circuit given in Figure 12.9
(a) Effective resistance of two 8 Ω resistors in the combination
(b) Current flowing through 4 Ω resistor
Solution: (a) Since, two $8 \Omega$ resistors are in parallel, then their effective resistance $R_{p}$ is given by$$\begin{aligned}\frac{1}{R_{p}}...
What is Joule’s heating effect? How can it be demonstrated experimentally? List its four applications in daily life.
Solution: According to the Joules heating effect, the amount of heat that a resistor produces is For the given resistor, the voltage is directly proportional to the square of the current.Resistance...
How will you conclude that the same potential difference (voltage) exists across three resistors connected in a parallel arrangement to a battery?
Solution: Create a circuit with three resistors (R1, R2, and R3) connected in parallel, as shown in the diagram below: Take a reading of the potential difference between three resistors that are...
How will you infer with the help of an experiment that the same current flows through every part of the circuit containing three resistances in series connected to a battery?
Solution: To construct the circuit, connect three resistors R1, R2, and R3 in series.Make use of an ammeter to observe the changes that have occurred in the current flow.Remove R1 and measure the...
What is electrical resistivity of a material? What is its unit? Describe an experiment to study the factors on which the resistance of conducting wire depends.
Answer: The ability of a conductor to resist the flow of electric current is an inherent property of the conductor itself. Each material's resistance is distinct from the others. Resistance is...
State Ohm’s law? How can it be verified experimentally? Does it hold good under all conditions? Comment.
Answer: Ohm’s law states that at constant temperature potential difference (voltage) across an ideal conductor is proportional to the current through it. V/I = R Ohm's law is being checked for...
Three incandescent bulbs of 100 W each are connected in series in an electric circuit. In another circuit, another set of three bulbs of the same wattage are connected in parallel to the same source.
(a) Will the bulb in the two circuits glow with the same brightness? Justify your answer.
(b) Now let one bulb in both the circuits get fused. Will the rest of the bulbs continue to glow in each circuit? Give reason.
Answer: a) In a series configuration, the resistance of the bulbs is three times greater than the resistance of a single bulb. As a result, when compared to the current in each bulb in the parallel...
B1 , B2, and B3 are three identical bulbs connected as shown in Figure 12.8. When all the three bulbs glow, a current of 3A is recorded by ammeter A.
What happens to the glow of the other two bulbs when the bulb B1 gets fused?
What happens to the reading of A1 , A2 , A3 and A when the bulb B2 gets fused?
Solution: In a parallel circuit, the potential difference does not become divided. As a result, when bulb one is fused, the glowing of the other bulbs will not be affected. 3 Amperes are being...
B1, B2, and B3 are three identical bulbs connected as shown in Figure 12.8. When all the three bulbs glow, a current of 3A is recorded by ammeter A. How much power is dissipated in the circuit when all the three bulbs glow together?
Solution: Evaluating the value, R= V/I = 4.5V/3A= 1.5Ω As we know that, substituting the value of parameters in P= I2R, we get = (3A)2 x 1.5 Ω = 13.5 W
Why is the parallel arrangement used in domestic wiring?
Answer: Parallel wiring is commonly used in domestic wiring because it ensures that the potential difference between each electrical appliance is the same across the board.
A current of 1 ampere flows in a series circuit containing an electric lamp and a conductor of 5 Ω when connected to a 10 V battery. Calculate the resistance of the electric lamp. Now if the resistance of 10 Ω is connected in parallel with this series combination, what change (if any) in current flowing through 5 Ω conductor and potential difference across the lamp will take place? Give reason.
Solution: Given: I= 1 A, V= 5 V 1) Let R be the resistance of the electric lamp. In series total resistance = 5 + R I = v/r 1 = 10/5+R R = 5 ohm 2) V across Lamp + conductor = 10 V V acoess Lamp =...
What is the commercial unit of electrical energy? Represent it in terms of joules.
Solution: Electrical energy is a term that is used to refer to energy that has been converted from electric potential energy when it is used informally. Commercial unit of electrical energy is...
What is electrical resistivity? In a series electrical circuit comprising a resistor made up of a metallic wire, the ammeter reads 5 A. The reading of the ammeter decreases to half when the length of the wire is doubled. Why?
Answer: The resistivity of a conductor is defined as the property of the conductor that prevents the flow of electric current. The resistance of a particular material is one-of-a-kind. Resistance is...
How does the use of a fuse wire protect electrical appliances?
Answer: The resistance of fuse wire is much higher than that of the main wiring. The electric current increases by a significant amount when this occurs. The fuse wire melts, causing the circuit to...
Draw a circuit diagram of an electric circuit containing a cell, a key, an ammeter, a resistor of 2 Ω in series with a combination of two resistors (4 Ω each) in parallel and a voltmeter across the parallel combination. Will the potential difference across the 2 Ω resistor be the same as that across the parallel combination of 4Ω resistors? Give reason.
Solution: The circuit diagram is: The following equation can be used to calculate the total resistance of a parallel combination of 40 resistors: $\frac{1}{R}=\frac{1}{4}+\frac{1}{4}=\frac{1}{2}$$R...
Should the resistance of an ammeter be low or high? Give reason.
Answer: The resistance of an ammeter should be low because the ammeter is connected in series with the circuit, and if the resistance of the circuit is too high, no current will flow through it.
Three 2 Ω resistors, A, B and C, are connected as shown in Figure 12.7. Each of them dissipates energy and can withstand a maximum power of 18W without melting. Find the maximum current that can flow through the three resistors?
Solution: We know that Current $P = I ^{2} R$ Evaluating the value,$$18 W = l ^{2} \times 2 \Omega \I ^{2}=18 W / 2 \Omega \ $$ =9 A I =3 AThis is the maximum current that can flow through the three...
A child has drawn the electric circuit to study Ohm’s law as shown in Figure 12.6. His teacher told that the circuit diagram needs correction. Study the circuit diagram and redraw it after making all corrections.
Solution: Specifically, the ammeter has been connected in parallel with the voltmeter, which is not the correct way to connect the two metres. The ammeter should be connected in series with the...
Unit of electric power may also be expressed as
(a) volt-ampere
(b) kilowatt-hour
(c) watt-second
(d) joule second
Solution: The answer is (a) volt-ampere The apparent power of an electrical circuit is measured in volt-amperes (VA), which is the unit of measurement. A watt-second (also spelled watt-second,...
Two resistors of resistance 2 Ω and 4 Ω when connected to a battery will have
(a) same current flowing through them when connected in parallel
(b) same current flowing through them when connected in series
(c) the same potential difference across them when connected in series
(d) different p
Solution: The answer is (b) same current flowing through them when connected in series When using a series combination, the current is not divided into branches because the resistor receives a...
An electric kettle consumes 1 kW of electric power when operated at 220 V. A fuse wire of what rating must be used for it?
(a) 1 A
(b) 2 A
(c) 4 A
(d) 5 A
Solution: The answer is (d) 5 A We know, P=V x I Substituting , 1000 w = 220v x I I =1000w/220v = 4.54 A = 5 A
In an electrical circuit, two resistors of 2 Ω and 4 Ω respectively are connected in series to a 6 V battery. The heat dissipated by the 4 Ω resistor in 5 s will be
(a) 5 J
(b) 10 J
(c) 20 J
(d) 30 J
Solution: Answer is (c) 20 J Equivalent resistance of the circuit is R = 4+2 = 6Ω current, I= V/R = 6/6= 1A the heat dissipated by 4-ohm resistor is, H = I2Rt = 20J
In an electrical circuit, three incandescent bulbs A, B and C of rating 40 W, 60 W and 100 W respectively are connected in parallel to an electric source. Which of the following is likely to happen regarding their brightness?
(a) The brightness of all the bulbs will be the same
(b) The brightness of bulb A will be the maximum
(c) The brightness of bulb B will be more than that of A
(d) The brightness of bulb C will be less than that of B
Solution: Answer is (c) Brightness of bulb B will be more than that of A Due to the fact that the bulbs are connected in parallel, their combined resistance will be less than the arithmetic sum of...
The resistivity does not change if
(a) the material is changed
(b) the temperature is changed
(c) the shape of the resistor is changed
(d) both material and temperature are changed
Solution: Answer is (c) the shape of the resistor is changed We will be re-arranging the terms we will receive, =A/RlAs a result, the resistivity of the material will be determined by its area and...
If the current I through a resistor is increased by 100% (assume that temperature remains unchanged), the increase in power dissipated will be
(a) 100 %
(b) 200 %
(c) 300 %
(d) 400 %
Solution: Answer is (c) 300 % The amount of heat generated by a resistor is proportional to the square of the current flowing through it. As a result, when the current is doubled, the dissipation of...
A student carries out an experiment and plots the V-I graph of three samples of nichrome wire with resistances R1, R2 and R3 respectively (Figure.12.5). Which of the following is true?
(a) R1 = R2 = R3
(b) R1 > R2 > R3
(c) R3 > R2 > R1
(d) R2 > R3 > R1
Solution: The answer is (c) R3 > R2 > R1 Resistance is inversely proportional to the amount of current flowing through it. Because the highest resistance will result in the least amount of current...
A cylindrical conductor of length l and uniform area of cross section A has resistance R. Another conductor of length 2l and resistance R of the same material has an area of cross-section
(a) A/2
(b) 3A/2
(c) 2A
(d) 3A
Solution: Answer is (c) 2A Explanation: We are well aware of this. R is equal to (L/A). The resistance of a wire of length L is denoted byThe area of the cross-section is denoted by the letter A.The...
Which of the following represents voltage?
(a)Work done/ Current×Time
(b) Work done × Charge
(c)Workdone×TimeCurrent
(d) Work done × Charge × Time
Solution: The answer is a) Explanation: Electric potential is the work done per unit charge. Electric Potential=Charge Work Done Again, Charge=Current×Time...
What is the minimum resistance which can be made using five resistors each of 1/5 Ω?
(a) 1/5 Ω
(b) 1/25 Ω
(c) 1/10 Ω
(d) 25 Ω
Solution: Answer is (b) 1/25 Ω Explanation: When resistors are connected in series, the resistance is reduced to a minimum. 1/R = 5 + 5 + 5 +5 +5= 25 Ω R=1/25Ω
The proper representation of the series combination of cells (Figure 12.4) obtaining maximum potential is
(a) (i)
(b) (ii)
(c) (iii)
(d) (iv)
Solution: The answer is (a) (i) Explanation: The positive terminal of the next cell is direct across from the negative terminal of the previous cell in this arrangement.
Identify the circuit (Figure 12.3) in which the electrical components have been properly connected.
(a) (i)
(b) (ii)
(c) (iii)
(d) (iv)
Solution: The answer is (b) (ii) Explanation: For current to flow, an electrical circuit must be closed. An open circuit can result from improperly connected components. An ammeter measures the...
A current of 1 A is drawn by a filament of an electric bulb. Number of electrons passing through a cross-section of the filament in 16 seconds would be roughly
(a) 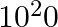
(b) 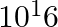
(c) 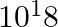
(d) 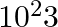
Solution: Answer is (a) 1020 Given $: I =1 A , t =16 s , e =1.6 \times 10^{-19} C$ Step 1: The filament draws current from the power supply. $I =\frac{ Q }{ t }=\frac{ Ne }{ t } \quad ($ Since $Q =...
Electrical resistivity of a given metallic wire depends upon
(a) its length
(b) its thickness
(c) its shape
(d) nature of the material
Solution: The answer is (d) nature of the material The resistivity of a material is an inherent property of the material. As an intensive property, it does not scale with the system's size, even if...
In the following circuits (Figure 12.2), the heat produced in the resistor or combination of resistors connected to a 12 V battery will be
(a) same in all the cases
(b) minimum in case (i)
(c) maximum in case(ii)
(d) maximum in case(iii)
Solution: The answer is (c) maximum in case(ii) In this example, two transistors are connected in series. Because they are connected in parallel, the total resistance in figure (iii) will be less...
A cell, a resistor, a key, and ammeter are arranged as shown in the circuit diagrams of Figure12.1. The current recorded in the ammeter will be
(a) maximum in (i)
(b) maximum in (ii)
(c) maximum in (iii)
(d) the same in all the cases
Solution: The answer is (d) the same in all the cases Because there are no modifications to any of the circuits, the current in all of the circuits will be the same.
Draw an appropriate schematic diagram showing common domestic circuits and discuss the importance of fuse. Why is it that a burnt-out fuse should be replaced by another fuse of identical rating?
Solution: Schematic diagram showing common domestic circuits The Importance of the Fuse A fuse is a type of safety device that is used in both household wiring and electrical appliance design.The...
Describe the working of an AC generator with the help of a labeled circuit diagram. What changes must be made in the arrangement to convert it to a DC generator?
Solution: It is stated in Faraday's law of electromagnetic induction that whenever a conductor moves in an electromagnetic field, an electromagnetic field is induced across the conductor. If a close...
Explain the phenomenon of electromagnetic induction. Describe an experiment to show that a current is set up in a closed loop when an external magnetic field passing through the loop increases or decreases.
Solution: The experimental diagram of circuit is: Electromagnetic induction is a phenomenon that occurs when a magnetic field is changed in a closed circuit, resulting in the generation of an...
Draw a labelled circuit diagram of a simple electric motor and explain its working. In what way these simple electric motors are different from commercial motors?
Solution: The schematic diagram of the simple electric motor is: A BCD coil is a coil that is placed between the two poles of a permanent magnet to create a magnetic field. The coil is positioned in...
Describe the activity that shows that a current-carrying conductor experiences a force perpendicular to its length and the external magnetic field. How does Fleming’s left-hand rule help us to find the direction of the force acting on the current-carrying conductor?
Answer: Take a small aluminium rod (AB) and bend it in half (of about 5 cm). As shown in Fig. 1, you can suspend it horizontally from a stand by using two connecting wires. A strong horseshoe magnet...
Explain with the help of a labeled diagram the distribution of magnetic field due to a current through a circular loop. Why is it that if a current-carrying coil has n turns the field produced at any point is n times as large as that produced by a single turn?
Answer: Even in a current-carrying loop, the right-hand thumb rule is observed and followed. This demonstrates that magnetic field lines are present all around the conducting wire. However, because...
With the help of a labeled circuit diagram illustrating the pattern of field lines of the magnetic field around a current-carrying straight long conducting wire. How is the right-hand thumb rule useful to find the direction of the magnetic field associated with a current-carrying conductor?
Answer: A straight conductor carrying current is held in the right hand with the thumb pointing in the direction of the current, according to the right-hand thumb rule. The fingers of the right hand...
Why does a magnetic compass needle pointing North and South in the absence of a nearby magnet get deflected when a bar magnet or a current-carrying loop is brought near it. Describe some salient features of magnetic lines of field concept.
Answer:Current carrying loops act as bar magnets with associated lines of the field, which are represented by the arrows. As a result, the existing earth's magnetic field is altered, resulting in a...
What is the role of fuse, used in series with any electrical appliance? Why should a fuse with a defined rating not be replaced by one with a larger rating?
Answer: An electrical fuse is composed of a thin wire of short length that is composed of tin and lead in the proportion of 75:25 percent. When the current exceeds the specified limit, the fuse...
What is the difference between a direct current and an alternating current? How many times does AC used in India change direction in one second?
Answer: The direction of current in an alternating current will change constantly, whereas the direction of current in a direct current will not change. In India, the air conditioner changes...
What is the role of the two conducting stationary brushes in a simple electric motor?
Answer: In order to power the motor, the two conducting stationary brushes must make contact with the outer sides of the two halves of the split rings, which are both insulated and attached to the...
Name four appliances wherein an electric motor, a rotating device that converts electrical energy to mechanical energy, is used as an important component. In what respect motors are different from generators?
Answer: Fans, mixers, washing machines, and computers are just a few examples of devices in which an electric motor, a rotating device that converts electrical energy to mechanical energy, plays a...
What does the divergence of magnetic field lines near the ends of a current-carrying straight solenoid indicate?
Answer: In a current-carrying straight solenoid, the divergence of magnetic field lines near the ends indicates a decrease in the strength of the magnetic field near and beyond the ends of the...
Meena draws magnetic field lines of the field close to the axis of a current-carrying circular loop. As she moves away from the centre of the circular loop she observes that the lines keep on diverging. How will you explain her observation?
Answer: The magnetic field strength decreases as the distance between the two points decreases. It is indicated by a decrease in the degree of closeness of the lines of field in the image.
What does the direction of thumb indicate in the right-hand thumb rule? In what way this rule is different from Fleming’s left-hand rule?
Answer: In the right-hand rule, the thumb held by curled fingers indicates the direction of current, whereas in Fleming's left-hand rule, the force experienced by a current-carrying conductor placed...
It is established that an electric current through a metallic conductor produces a magnetic field around it. Is there a similar magnetic field produced around a thin beam of moving (i) alpha particles, (ii) neutrons? Justify your answer.
Solution: i) Due to the fact that they are positively charged, alpha particles serve to generate current in the direction of motion. ii) Because neutrons do not have any charge on them, they do not...
A magnetic compass shows a deflection when placed near a current-carrying wire. How will the deflection of the compass get affected if the current in the wire is increased? Support your answer with a reason.
Answer: As the deflection increases, so does the deflection. The strength of the magnetic field will be directly proportional to the amount of current that passes through the straight conductor, as...
AB is a current-carrying conductor in the plane of the paper as shown in Figure 13.7. What are the directions of magnetic fields produced by it at points P and Q? Given r1 > r2, where will the strength of the magnetic field be larger?
Solution: P puts you into the plane of paper, and Q takes you out of it. The strength of the magnetic field is greater at the point that is closer to the source, which is Q.
Under what conditions permanent electromagnet is obtained if a current-carrying solenoid is used? Support your answer with the help of a labeled circuit diagram.
Solution: It is necessary to meet the following conditions in order to obtain a permanent electromagnet from a current-carrying solenoid. Soft iron is used in a closed-circuit core.
A magnetic compass needle is placed in the plane of paper near point A as shown in Figure 13.6. In which plane should a straight current-carrying conductor be placed so that it passes through A and there is no change in the deflection of the compass? Under what condition is the deflection maximum and why?
Answer: In the plane of the paper itself, to be precise. The axis of the compass is vertical, and the magnetic field produced by the conductor is vertical as well. Because of this, it is not...
The most important safety method used for protecting home appliances from short-circuiting or overloading is
(a) earthing
(b) use of fuse
(c) use of stabilizers
(d) use of electric meter
Solution: Answer is (b) use of fuse An electrical fuse is composed of a thin wire of short length that is composed of tin and lead in the proportion of 75:25 percent. When the current exceeds the...
To convert an AC generator into DC generator
(a) split-ring type commutator must be used
(b) slip rings and brushes must be used
(c) a stronger magnetic field must be used
(d) a rectangular wire loop must be used
Solution: Answer is (a) split-ring type commutator must be used After each half-turn of the armature, a split-ring type commutator reverses the direction of the current flowing through it. This...
The strength of the magnetic field inside a long current carrying straight solenoid is
(a) more at the ends than at the centre
(b) minimum in the middle
(c) same at all points
(d) found to increase from one end to the other
Solution: Answer is (c) same at all points Magnetic field lines are parallel inside the solenoid. This indicates the existence of a strong magnetic field. As a result, the magnetic field is uniform...
A constant current flow in a horizontal wire in the plane of the paper from east to west as shown in Figure 13.5. The direction of the magnetic field at a point will be North to South
(a) directly above the wire
(b) directly below the wire
(c) at a point located in the plane of the paper, on the north side of the wire
(d) at a point located in the plane of the paper, on the south side of the wire
Solution: The answer is (b) directly below the wire We can determine the direction of the magnetic field beneath the wire by applying the right-hand thumb rule.
Choose the incorrect statement
(a) Fleming’s right-hand rule is a simple rule to know the direction of induced current
(b) The right-hand thumb rule is used to find the direction of magnetic fields due to current-carrying conductors
(c) The difference between the direct and alternating currents is that the direct current always flows in one direction, whereas the alternating current reverses its direction periodically
(d) In India, the AC changes direction after every 1/50 second
Solution: The answer is (d) In India, the AC changes direction after every 1/50 second In India, the alternating current frequency is 50 Hz. Each cycle, the direction changes twice, so that the...
In the arrangement shown in Figure 13.4, there are two coils wound on a non-conducting cylindrical rod. Initially, the key is not inserted. Then the key is inserted and later removed. Then
(a) the deflection in the galvanometer remains zero throughout
(b) there is a momentary deflection in the galvanometer, but it dies out shortly and there is no effect when the key is removed
(c) there are momentary galvanometer deflections that die out shortly; the deflections are in the same direction
(d) there are momentary galvanometer deflections that die out shortly; the deflections are in opposite directions
Solution: The answer is (d) there are momentary galvanometer deflections that die out shortly; the deflections are in opposite directions When the key is plugged in, the galvanometer shows...
Commercial electric motors do not use
(a) an electromagnet to rotate the armature
(b) effectively large number of turns of conducting wire in the current-carrying coil
(c) a permanent magnet to rotate the armature
(d) a soft iron core on which the coil is wound
Solution: Answer is (c) a permanent magnet to rotate the armature Electromagnets are used in place of permanent magnets in electric motors.
A uniform magnetic field exists in the plane of paper pointing from left to right as shown in Figure 13.3. In the field an electron and a proton move as shown. The electron and the proton experience
(a) forces both pointing into the plane of paper
(b) forces both pointing out of the plane of paper
(c) forces pointing into the plane of paper and out of the plane of paper, respectively
(d) force pointing opposite and along the direction of the uniform magnetic field respectively
Solution: Answer is (a) forces both pointing into the plane of the paper Explanation: The direction of an electron's motion is the polar opposite of the direction of an electric current. This will...
For a current in a long straight solenoid N- and S-poles are created at the two ends. Among the following statements, the incorrect statement is
(a) The field lines inside the solenoid are in the form of straight lines which indicates that the magnetic field is the same at all points inside the solenoid
(b) The strong magnetic field produced inside the solenoid can be used to magnetise a piece of magnetic material like soft iron, when placed inside the coil
(c) The pattern of the magnetic field associated with the solenoid is different from the pattern of the magnetic field around a bar magnet
(d) The N- and S-poles exchange position when the direction of current through the solenoid is reversed
Solution: Answer is (c) The pattern of the magnetic field associated with the solenoid is different from the pattern of the magnetic field around a bar magnet Because the solenoid behaves similarly...
For a current in a long straight solenoid N- and S-poles are created at the two ends. Among the following statements, the incorrect statement is
(a) The field lines inside the solenoid are in the form of straight lines which indicates that the magnetic field is the same at all points inside the solenoid
(b) The strong magnetic field produced inside the solenoid can be used to magnetize a piece of a magnetic material like soft iron when placed inside the coil
(c) The pattern of the magnetic field associated with the solenoid is different from the pattern of the magnetic field around a bar magnet
(d) The N- and S-poles exchange position when the direction of current through the solenoid is reversed
Solution: Answer is (c) The pattern of the magnetic field associated with the solenoid is different from the pattern of the magnetic field around a bar magnet Explanation: Due to the fact that a...
A circular loop placed in a plane perpendicular to the plane of paper carries a current when the key is ON. The current as seen from points A and B (in the plane of the paper and on the axis of the coil) is anti-clockwise and clockwise respectively. The magnetic field lines point from B to A. The N-pole of the resultant magnet is on the face close to
(a) A
(b) B
(c) A if the current is small, and B if the current is large
(d) B if the current is small and A if the current is large
Solution: Answer is (a) A Explanation: The magnetic field will be directed from the south pole to the north pole. As a result of field lines pointing from point B to point A, point A is displaying...
Choose the incorrect statement from the following regarding magnetic lines of the field
(a) The direction of the magnetic field at a point is taken to be the direction in which the north pole of a magnetic compass needle points
(b) Magnetic field lines are closed curves
(c) If magnetic field lines are parallel and equidistant, they represent zero-field strength
(d) The relative strength of the magnetic field is shown by the degree of closeness of the field lines
In the case of open magnetic field lines drawn over the horizontal plane ABCD, the magnetic field lines will take the shape of concentric circles, with the centre of each circle located at the axis...
A light ray enters from medium A to medium B as shown in the Figure. The refractive
index of medium B relative to A will be (a) greater than unity (b) less than unity (c) equal to unity (d) zero Answer: (a) greater than unity Explanation: The refractive index is the bending of a...
Under which of the following conditions a concave mirror can form an image larger than the actual object?
(a) When the object is kept at a distance equal to its radius of curvature (b) When an object is kept at a distance less than its focal length (c) When an object is placed between the focus and...
A 10 mm long awl pin is placed vertically in front of a concave mirror. A 5 mm long image of the awl pin is formed at 30 cm in front of the mirror. The focal length of this mirror is
(a) – 30 cm (b) – 20 cm (c) – 40 cm (d) – 60 cm Answer: (b) – 20 cm Explanation: In this case, the focal length of the concave mirror is – 20 cm.
Choose the incorrect statement from the following regarding magnetic lines of the field
(a) The direction of the magnetic field at a point is taken to be the direction in which the north pole of a magnetic compass needle points
(b) Magnetic field lines are closed curves
(c) If magnetic field lines are parallel and equidistant, they represent zero-field strength
(d) The relative strength of the magnetic field is shown by the degree of closeness of the field lines
Answer is (c) If magnetic field lines are parallel and equidistant, they represent zero-field strength Explanation: Due to the fact that parallel lines of magnetism represent a homogenous magnetic...
Explain the phenomenon of dispersion of white light through a glass prism, using a suitable ray diagram.
Answer: When a ray of light enters a prism, it bends because of refraction of light. When the ray of light finally emerges out of the prism. it deviates drastically from its original path. This...
How can we explain the reddish appearance of the sun at sunrise or sunset? Why does it not appear red at noon?
Answer: The sun appears red at sunrise and sunset because the sun is closer to the horizon during these times of the year. During its journey to our eyes, sunlight passes through a denser layer of...
Explain the refraction of light through a triangular glass prism using a labelled ray diagram. Hence define the angle of deviation.
The refraction of light through a triangular glass prism is depicted in the illustration above. At the first surface AB, a beam of light PE is penetrating the glass from the surrounding air. Due to...
When do we consider a person to be myopic or hypermetropic? Explain using diagrams how the defects associated with myopic and hypermetropic eye can be corrected?
Answer: Myopia is a condition in which a person's ability to see distant objects clearly is impaired. Myopia is a condition in which the image is created in front of the retina rather than behind...
Explain the structure and functioning of the human eye. How are we able to see nearby as well as distant objects?
Answer: The human eye is considered to be one of the most valuable and sensitive sense organs on the planet. It allows us to take in the beauty of the world and the colours that surround us. After...
What is the difference in colours of the Sun observed during sunrise/sunset and noon? Give an explanation for each.
Answer: The sun appears red at sunrise and sunset because the sun is closer to the horizon during these times of the year. During its journey to our eyes, sunlight passes through a denser layer of...
Why is the colour of the clear sky blue?
Answer: The colour blue is the one that scatters the most light in the visible spectrum, and it is the most intense. As a result, the blue colour is able to reach us and the sky appears to be blue.
Why do we see a rainbow in the sky only after rainfall?
Answer: Rainbows are generated by the dispersion of sunlight by microscopic water droplets in the atmosphere, which are present in the air. A rainbow is always generated in the opposite direction to...
Is the position of a star as seen by us its true position? Justify your answer.
Answer: When starlight enters the earth's atmosphere, it is subjected to constant refraction. Refraction happens in a material with a refractive index that is gradually changing. Starlight is...
Draw a ray diagram showing the dispersion through a prism when a narrow beam of white light is incident on one of its refracting surfaces. Also indicate the order of the colours of the spectrum obtained.
Solution: The ray diagram is:
How will you use two identical prisms so that a narrow beam of white light incident on one prism emerges out of the second prism as white light? Draw the diagram.
Solution: Using two identical prisms that are inverted with regard to one another, we can create a narrow beam of white light that is incident on one prism and emerges out of the second prism as...
A person needs a lens of power –4.5 D for correction of her vision. (c) What is the nature of the corrective lens?
Answer: Myopia is a condition in which a pupil is unable to see the chalkboard that is far away from her. As a result, the doctor recommends a concave lens with a proper focal length. Negative sign...
A person needs a lens of power –4.5 D for correction of her vision.
(a) What kind of defect in vision is she suffering from?
(b) What is the focal length of the corrective lens?
Solution: Myopia is a condition in which a pupil is unable to see the chalkboard that is far away from her. As a result, the doctor recommends a concave lens with a proper focal length. Answer is...
How are we able to see nearby and also the distant objects clearly?
Answer: By varying the focal length of the lens, we can make our eyes focus on images from a variety of different distances. The action of the Ciliary muscle aids in the adjustment of the focal...
A student sitting at the back of the classroom cannot read clearly the letters written on the blackboard. What advice will a doctor give to her? Draw ray diagram for the correction of this defect.
Myopia is a condition in which a pupil is unable to see the chalkboard that is far away from her. As a result, the doctor recommends a concave lens with a proper focal length.
Draw ray diagrams each showing (i) myopic eye and (ii) hypermetropic eye.
Solution: Myopia is a condition in which a person's ability to see distant objects clearly outweighs his ability to perceive those closer to him. Hypermetropia is a condition in which a person...
Which of the following statement is correct?
(a) A person with myopia can see distant objects clearly
(b) A person with hypermetropia can see nearby objects clearly
(c) A person with myopia can see nearby objects clearly
(d) A person with hypermetropia cannot see distant objects clearly
Solution: Answer is (c) A person with myopia can see nearby objects clearly Myopia is a condition in which a person's ability to see distant objects clearly outweighs his ability to perceive those...
The focal length of the eye lens increases when eye muscles
(a) are relaxed and the lens becomes thinner
(b) contract and lens become thicker
(c) are relaxed and the lens becomes thicker
(d) contract and lens become thinner
Solution: Answer is (a) are relaxed and the lens becomes thinner When the eye muscles relax and become thinner, the focal length of the eye lens grows in proportion to its thickness. The retina is...
When light rays enter the eye, most of the refraction occurs at the
(a) crystalline lens
(b) outer surface of the cornea
(c) iris
(d) pupil
Solution: The answer is (b) the outer surface of the cornea The cornea is a thin membrane that allows light to pass through to the eye. When light rays strike the cornea, they are bent inward and...
The bluish colour of water in the deep sea is due to
(a) the presence of algae and other plants found in water
(b) reflection of sky in water
(c) scattering of light
(d) absorption of light by the sea
Solution: The answer is (b) reflection of sky in water Explanation: Water is colourless, but it takes on the colour of whatever it is reflected by. As a result, the sea seems blue.
Which of the following phenomena contributes significantly to the reddish appearance of the sun at sunrise or sunset?
(a) Dispersion of light
(b) Scattering of light
(c) Total internal reflection of light
(d) Reflection of light from the earth
Solution: The answer is (b) Scattering of light Explanation: Because red scatters the least amount of light, it may go a long distance. When the sun sets or rises, light has to travel a long...
The danger signals installed at the top of tall buildings are red in colour. These can be easily seen from a distance because among all other colours, the red light
(a) is scattered the most by smoke or fog
(b) is scattered the least by smoke or fog
(c) is absorbed the most by smoke or fog
(d) moves fastest in air
Solution: The answer is (b) is scattered the least by smoke or fog Because the wavelength of red colour is the longest, it may be seen clearly from a long distance. It is the colour that is least...
Which of the following statements is correct regarding the propagation of light of different colours of white light in air?
(a) Red light moves fastest
(b) Blue light moves faster than green light
(c) All the colours of the white light move with the same speed
(d) Yellow light moves with the mean speed as that of the red and the violet light
Solution: The correct option is C All of the colours in the white light travel at the same speed as one another. Because air is not a dispersive medium, light of all wavelengths travels at the same...
Twinkling of stars is due to atmospheric
(a) dispersion of light by water droplets
(b) refraction of light by different layers of varying refractive indices
(c) scattering of light by dust particles
(d) internal reflection of light by clouds
Solution: The answer is (b) refraction of light by different layers of varying refractive indices Explanation: The refraction of light maintains the change in the position of the source of light....
The clear sky appears blue because
(a) blue light gets absorbed in the atmosphere
(b) ultraviolet radiations are absorbed in the atmosphere
(c) violet and blue lights get scattered more than lights of all other colors by the atmosphere
(d) light of all other colors is scattered more than the violet and blue color lights by the atmosphere
Solution: Answer is (c) violet and blue lights get scattered more than lights of all other colors by the atmosphere. Rayleigh's scattering of sunlight is responsible for the blue appearance of the...
At noon the sun appears white as
(a) light is least scattered
b) all the colours of the white light are scattered away
(c) blue colour is scattered the most
(d) red colour is scattered the most
Solution: The answer is (b) all the colours of the white light are scattered away This is due to the dispersion of light caused by the presence of air in the environment.
Which of the following phenomena of light are involved in the formation of a rainbow?
(a) Reflection, refraction, and dispersion
(b) Refraction, dispersion, and total internal reflection
(c) Refraction, dispersion, and internal reflection
d) Dispersion, scattering and total internal reflection
Solution: Answer is (c) Refraction, dispersion, and internal reflection Dispersion of light results in the scattering of white light into multiple colours at different angles, resulting in internal...
A prism ABC (with BC as a base) is placed in different orientations. A narrow beam of white light is incident on the prism as shown in Figure 11.1. In which of the following cases, after dispersion, the third colour from the top corresponds to the colour of the sky? The Human Eye and the Colourful World CHAPTER11 Fig.11.1
(a) (i)
(b) (ii)
(c) (iii)
(d) (iv)
Solution: The answer is (b) (ii) If the prism is kept with BC at the bottom, a band of colour appears at the bottom that is violet. If the prism is kept with BC at the top, violet will be at the top...
A student sitting on the last bench can read the letters written on the blackboard but is not able to read the letters written in his textbook. Which of the following statements is correct?
(a) The near point of his eyes has receded away
(b) The near point of his eyes has come closer to him
(c) The far point of his eyes has come closer to him
(d) The far point of his eyes has receded away
Solution: Answer is (a) The near point of his eyes has receded away In hypermetropia, the near spot of the eye is moved away for 25 cm. Thus, in order to read properly, the user needs maintain the...
A person cannot see distinctly objects kept beyond 2 m. This defect can be corrected by using a lens of power
(a) + 0.5 D
(b) – 0.5 D
(c) + 0.2 D
(d) – 0.2 D
Solution:The answer is (b) – 0.5 D Because the individual is myopic and in need of a concave mirror, the power would be in the negative. P=1/f=1/2m=0.5 D.
An element X of group 15 exists as a diatomic molecule and combines with hydrogen at 773 K in presence of the catalyst to form a compound, ammonia which has a characteristic pungent smell.
(a) Identify the element X. How many valence electrons does it have?
(b) Draw the electron dot structure of the diatomic molecule of X. What type of bond is formed in it?
Solution: Nitrogen is the correct answer, as it possesses five electrons in its outermost shell.
An element X of group 15 exists as a diatomic molecule and combines with hydrogen at 773 K in presence of the catalyst to form a compound, ammonia which has a characteristic pungent smell.
(c) Draw the electron dot structure for ammonia and what type of bond is formed in it?
Solution: Covalent bonding is formed by ammonia.
An element X which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants.
(e) Locate the position of the element in the Modern Periodic Table
Solution: The position of sulfur is Group 16 Period 3.
An element X which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants.
(c) Write the balanced chemical equation for the thermal decomposition of ferrous sulphate crystals?
(d) What would be the nature (acidic/ basic) of oxides formed?
Solution: 2FeSO4 Fe2O3+SO2+SO3Sulphur oxides are acidic in nature
An element X which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants.
(a) Identify the element X
(b) Write the electronic configuration of X.
Solution: Element X is Sulphur2,8,6
Electropositive nature of the element(s) increases down the group and decreases across the period
(b) Electronegativity of the element decreases down the group and increases across the period
(c) Atomic size increases down the group and decreases across a period (left to right)
(d) Metallic character increases down the group and decreases across a period.
Based on the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
(c) Name the element with the smallest atomic size
(d) Name the element which is a metalloid
Solution: (a)The electropositive character of the element(s) increases as it progresses through the group and diminishes as it progresses through time. (b) The element's electronegativity drops as...
Electropositive nature of the element(s) increases down the group and decreases across the period
(b) Electronegativity of the element decreases down the group and increases across the period
(c) Atomic size increases down the group and decreases across a period (left to right)
(d) Metallic character increases down the group and decreases across a period.
Based on the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
(e) Name the element which shows maximum valency.
Solution: (a)The electropositive character of the element(s) increases as it progresses through the group and diminishes as it progresses through time. (b) The element's electronegativity drops as...
Electropositive nature of the element(s) increases down the group and decreases across the period
(b) Electronegativity of the element decreases down the group and increases across the period
(c) Atomic size increases down the group and decreases across a period (left to right)
(d) Metallic character increases down the group and decreases across a period.
Based on the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
(a) Name the most electropositive element among them
(b) Name the most electronegative element
Solution: (a)The electropositive character of the element(s) increases as it progresses through the group and diminishes as it progresses through time. (b) The element's electronegativity drops as...
Mendeleev ′ predicted the existence of certain elements not known at that time and named two of them as Eka-silicon and Eka-aluminium.
(c) Classify these elements as metals, non-metals, or metalloids
(d) How many valence electrons are present in each one of them?
Solution: c) Gallium-Group 13 Period 4 is a periodic table of elements. The elements germanium and gallium are both metalloids; however, gallium is a metal. d) Germanium contains four electrons, but...
Mendeleev ′ predicted the existence of certain elements not known at that time and named two of them as Eka-silicon and Eka-aluminium.
(a) Name the elements which have taken the place of these elements
(b) Mention the group and the period of these elements in the Modern Periodic Table.
Solution: a) Eka-Silicon was replaced by germanium, while Eka-Aluminum was replaced by gallium. b) Gallium was replaced by germanium. b) Germanium-Group 14 Period 4 (Germanium-Group 14).
(a) In this ladder (Figure 5.2) symbols of elements are jumbled up. Rearrange these symbols of elements in the increasing order of their atomic number in the Periodic Table.
(b) Arrange them in the order of their group also.
Solution: a) H, He, Li, Be, B, C, N, O, F, Ne, Mg, Al Si, P, S, Cl, Ar, K, Ca b) Group 1:H, Li, Na, K Group 2: Be, Mg, Ca Group 13: B. Al Group 14: C, Si Group 15: N. P Group 16: 0, S Group 17: F. U...
Complete the following crossword puzzle in figure:
Across:
(1) An element with atomic number 12.
(3) Metal used in making cans and member of Group 14.
(4) A lustrous non-metal which has 7 electrons in its outermost shell.
Down:
(2) Highly reactive and soft metal which imparts yellow colour when subjected to flame and is kept in kerosene.
(5) The first element of the second Period
(6) An element which is used in making fluorescent bulbs and is the second member of Group 18 in the Modern Periodic Table
(7) A radioactive element which is the last member of the halogen family.
(8) Metal which is an important constituent of steel and forms rust when exposed to moist air.
(9) The first metalloid in Modern Periodic Table whose fibres are used in making bullet-proof vests
Solution: Across 1) Magnesium 3) Tin 4) Iodine Down 2) Sodium 5) Lithium 6) Neon 7) Astatine 8) Iron 9) Boron
Atomic number of a few elements are given below 10, 20, 7, 14
(c) Identify the Periods of these elements in the Periodic Table
(d) What would be the electronic configuration for each of these elements?
Solution: (c)Nitrogen and Neon belong to period 2. Calcium and silicon belongs to Period 3. (d)Electronic Configurations Neon-2,8 Calcium-2,8,8,2 Nitrogen-2,5 Silicon-2,8,4
