Answer: It possesses less electrons than the parent atom, but the overall nuclear charge remains the same, resulting in greater attraction of electrons to the nucleus. Cations have smaller radii...
Consider the following species : 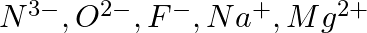 , and
, and 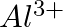 (a) What is common in them? (b) Arrange them in the order of increasing ionic radii.
(a) What is common in them? (b) Arrange them in the order of increasing ionic radii.
Answer: (a) All of the species have the same number of electrons, i.e. 10 electrons. They are, therefore, isoelectronic species. (b) The following is the arrangement of the given ions in ascending...
How does H2 O2 act as a dying specialist?
Solution: Hydrogen peroxide goes about as a solid oxidizing specialist both in fundamental and acidic media. When added to a material, it breaks the substance obligations of the chromophores...
Knowing the properties of H2O and D2O, do you imagine that D2O can be utilized for drinking purposes?
Solution: D2O is referred to as weighty water which goes about as an arbitrator (dials back the pace of response). Because of this property, it can't be utilized for drinking reason since it dials...
Portray the helpfulness of water in biosphere and organic frameworks.
Solution: Water is extremely important for all types of life which establish 65% of human body and 95% of plants.it assumes an indispensable part in the biosphere because of its → Thermal...
Is demineralised or refined water valuable for drinking purposes? If not, how might it be made valuable?
Solution: Water is extremely fundamental for our life. It comprises of many broke down supplements that are needed for ourselves and furthermore for plants and creatures. Demineralised water is...
What is implied by ‘demineralised’ water and how might it be acquired ?
Solution: This water is liberated from every one of the solvent mineral salts and it doesn't contain any cation or anion. It is acquired progressively by going the water through anion trade and...
What causes the impermanent and super durable hardness of water?
Solution: Because of the presence of dissolvable salts of magnesium and calcium as chlorides in water, hardness stays super durable in water. Because of the presence of dissolvable salts of calcium...
Portray the construction of the normal type of ice.
Solution: For the most part, ice is the translucent type of water. It visibles in a hexagonal structure in case it is solidified at climatic strain. At the point when the temperature is...
Analyze the designs of H2O and H2 O2.
Solution: The water atom will be shown with a bond point of 104 .5o has a bowed structure in vaporous stage. The O-H bond length is 95.7 pm. Structure : Hydrogen peroxide has a non-planar design...
Organize the accompanying NaH, MgH2 and H2O arranged by expanding diminishing property.
Solution: Ionic hydrides are solid diminishing specialists. NaH can undoubtedly give its electrons. Henceforth, it is most diminishing in nature. Both, MgH2 and H2O are covalent hydrides. H2O is...
Organize the accompanying H–H, D–D and F–F arranged by expanding bond separation enthalpy.
Solution: The bond pair in D–D bond is more emphatically drawn in by the core than the bond pair in H–H bond. This is a direct result of the greater atomic mass of D2. The more grounded the...
Organize the accompanying LiH, NaH and CsH arranged by expanding ionic person.
Solution: The ionic person of a bond is reliant upon the electro negativities of the molecules in question. The higher the distinction between the electro negativities of molecules, the more modest...
Organize the accompanying CaH2, BeH2 and TiH2 arranged by expanding electrical conductance.
Solution: The electrical conductance of an atom chiefly relies upon its covalent or ionic nature. CaH2 is an ionic hydride, which conducts power in the liquid state. Titanium hydride, TiH2 is...
Among NH3, H2O and HF, which would you hope to have most noteworthy greatness of hydrogen holding and why?
Solution: The degree of hydrogen holding predominantly relies upon (I) Electronegativity (ii) Number of hydrogen iotas accessible for holding. Among oxygen, fluorine and nitrogen, the expanding...
How does the nuclear hydrogen or oxy-hydrogen light capacity for cutting and welding purposes ? Clarify.
Solution: The nuclear hydrogen light is otherwise called oxy-hydrogen light. These molecules are delivered through dihydrogen separation with the assistance of an electric circular segment which...
How would you anticipate that the metallic hydrides should be valuable for hydrogen stockpiling? Clarify
Solution: Metallic hydrides are hydrogen inadequate. They don't keep the law of consistent structure. It has been set up that in the hydrides of Pd, Ac, Ni, and Ce, hydrogen possesses the...
What do you comprehend by the expression “non-stoichiometric hydrides”? Do you anticipate that this type of the hydrides should be framed by soluble base metals? Legitimize your reply.
Solution: Non-Stoichiometric hydrides are hydrogen-insufficient mixtures which is framed by the response of dihydrogen with d-square and f-block components. These hydrides don't observe the law of...
Do you expect the carbon hydrides of the sort (Cn H2n+2 ) to go about as ‘Lewis’ base or corrosive? Legitimize.
Solution: For carbon hydrides which have a place with type (Cn H2n+2), the accompanying hydrides are feasible for \[\begin{array}{*{35}{l}} n\text{ }=\text{ }1\Rightarrow CH4 \\ ~ \\...
What do you comprehend by (I) electron rich – mixtures of hydrogen, (ii) electron-exact, and (iii) electron-lacking? Give avocation appropriate models.
Solution: Sub-atomic hydride is grouped based on the presence of the bonds and absolute number of electrons in their Lewis structures as: Electron-inadequate hydrides Electron-exact hydrides...
Talk about the results of high enthalpy of H–H bond as far as synthetic reactivity of dihydrogen
Solution: The ionization enthalpy of H–H bond is higher (1312 kJ mol–1 ) which shows that hydrogen has a low propensity to frame H+ particles. Its ionization enthalpy esteem is equivalent to that of...
For what reason does hydrogen happen in a diatomic structure instead of in a monoatomic structure under ordinary conditions?
Solution: The ionization enthalpy of hydrogen molecule is higher. In this manner, it is more enthusiastically to eliminate its electron. This outcomes its propensity to exist in the low monoatomic...
Legitimize the situation of hydrogen in the intermittent table based on its electronic design.
Solution: The first component in the occasional table is hydrogen. Hydrogen shows double conduct since it has just 1 electron on its one 'S' shell.(i.e.,) hydrogen takes after the two incandescent...
Work out the entropy change in environmental elements when 1.00 mol of H2O(l) is shaped under standard conditions. ![Rendered by QuickLaTeX.com \[f\text{ }H0=\text{ }\text{ }286\text{ }kJ\text{ }mol1.\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0876268cab5e7e19237ca8d099603274_l3.png)
Solution: It is given that 286 kJ mol–1 of warmth is advanced on the development of 1 mol of H2O(l). Accordingly, an equivalent measure of warmth will be consumed by the environmental elements....
Comment on the thermodynamic stability of NO(g), given
Solution: The positive worth of ∆rH demonstrates that warmth is assimilated during the development of NO(g). This implies that NO(g) has higher energy than the reactants (N2 and O2)....
The harmony steady for a response is 10. What will be the worth of ![Rendered by QuickLaTeX.com \[G0?\text{ }R\text{ }=\text{ }8.314\text{ }JK1\text{ }mol1,\text{ }T\text{ }=\text{ }300\text{ }K.\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c5355a31e6d1d5f6327e806c31f733f0_l3.png)
Solution: From the articulation, \[\begin{array}{*{35}{l}} G\theta \text{ }=\text{ }\text{ }2.303\text{ }RT\text{ }logKeq \\ ~ \\ G\theta \text{ }for\text{ }the\text{ }response,\text{ }=\text{...
For the response ![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} 2A\left( g \right)\text{ }+\text{ }B\left( g \right)\text{ }\to \text{ }2D\left( g \right) \\ ~ \\ U\theta \text{ }=\text{ }\text{ }10.5\text{ }kJ\text{ }and\text{ }S\theta \text{ }=\text{ }\text{ }44.1\text{ }JK1\text{ }. \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-cbc82328853f02bdc578b87abcbd9b11_l3.png)
Compute ∆Gθ for the response, and anticipate whether the response might happen suddenly.
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} 2A\left( g \right)\text{ }+\text{ }B\left( g \right)\text{ }\to \text{ }2D\left( g \right) \\ ~ \\ U\theta \text{ }=\text{ }\text{ }10.5\text{ }kJ\text{ }and\text{ }S\theta \text{ }=\text{ }\text{ }44.1\text{ }JK1\text{ }. \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-cbc82328853f02bdc578b87abcbd9b11_l3.png)
Solution: For the given response, \[\begin{array}{*{35}{l}} 2\text{ }A\left( g \right)\text{ }+\text{ }B\left( g \right)\text{ }\to \text{ }2D\left( g \right) \\ ~ \\ ng\text{ }=\text{...
For the response, 2Cl(g) → Cl2(g), what are the indications of ∆H and ∆S ?
Solution: ∆H and ∆S are negative The given response addresses the development of chlorine particle from chlorine molecules. Here, bond development is occurring. Along these lines, energy is being...
For the response at 298 K, ![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} 2A\text{ }+\text{ }B\text{ }\to \text{ }C\text{ }H\text{ }=\text{ }400\text{ }kJ\text{ }mol1 \\ ~ \\ what's\text{ }more,\text{ }S\text{ }=\text{ }0.2\text{ }kJ\text{ }K1\text{ }mol1 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-dda209e4073843cae616fbc6002155e5_l3.png)
At what temperature will the response become unconstrained believing ∆H and ∆S to be consistent over the temperature range?
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} 2A\text{ }+\text{ }B\text{ }\to \text{ }C\text{ }H\text{ }=\text{ }400\text{ }kJ\text{ }mol1 \\ ~ \\ what's\text{ }more,\text{ }S\text{ }=\text{ }0.2\text{ }kJ\text{ }K1\text{ }mol1 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-dda209e4073843cae616fbc6002155e5_l3.png)
Solution: From the articulation, \[G\text{ }=\text{ }H\text{ }\text{ }TS\] Expecting the response at balance, ∆T for the response would be: \[\begin{array}{*{35}{l}} \left( G\text{...
For a segregated framework, ![Rendered by QuickLaTeX.com \[U\text{ }=\text{ }0\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-e877d1035c3edbb47c89fe48897ae3da_l3.png)
what will be ∆S?
Solution: ∆S will be positive i.e., more noteworthy than nothing \[Since\text{ }U\text{ }=\text{ }0,\] ∆S will be positive and the response will be unconstrained.
Ascertain the enthalpy change for the interaction ![Rendered by QuickLaTeX.com \[CCl4\left( g \right)\text{ }\to \text{ }C\left( g \right)\text{ }+\text{ }4Cl\left( g \right)\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-2f50a987d1e8f6ebc394f6ed04c1c2b4_l3.png)
furthermore, ascertain bond enthalpy of C–Cl in CCl4(g). ![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} vapH\theta \text{ }\left( CCl4 \right)\text{ }=\text{ }30.5\text{ }kJ\text{ }mol1\text{ }. \\ ~ \\ fH\theta \text{ }\left( CCl4 \right)\text{ }=\text{ }\text{ }135.5\text{ }kJ\text{ }mol1\text{ }. \\ ~ \\ aH\theta \text{ }\left( C \right)\text{ }=\text{ }715.0\text{ }kJ\text{ }mol1\text{ }, \\ ~ \\ where\text{ }aH\theta \text{ }is\text{ }enthalpy\text{ }of\text{ }atomisation \\ ~ \\ aH\theta \text{ }\left( Cl2 \right)\text{ }=\text{ }242\text{ }kJ\text{ }mol1 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-183416d42bdde6938df21980fb24335b_l3.png)
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} vapH\theta \text{ }\left( CCl4 \right)\text{ }=\text{ }30.5\text{ }kJ\text{ }mol1\text{ }. \\ ~ \\ fH\theta \text{ }\left( CCl4 \right)\text{ }=\text{ }\text{ }135.5\text{ }kJ\text{ }mol1\text{ }. \\ ~ \\ aH\theta \text{ }\left( C \right)\text{ }=\text{ }715.0\text{ }kJ\text{ }mol1\text{ }, \\ ~ \\ where\text{ }aH\theta \text{ }is\text{ }enthalpy\text{ }of\text{ }atomisation \\ ~ \\ aH\theta \text{ }\left( Cl2 \right)\text{ }=\text{ }242\text{ }kJ\text{ }mol1 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-183416d42bdde6938df21980fb24335b_l3.png)
Solution: The synthetic conditions suggesting to the given upsides of enthalpies are:
Calculate the standard enthalpy of formation of CH3OH(l) from the following data:
Solution: The response that happens during the arrangement of CH3OH(l) can be composed as: \[C\left( s \right)\text{ }+\text{ }2H2O\left( g \right)\text{ }+\text{ }\left( 1/2...
Given ![Rendered by QuickLaTeX.com \[N2\text{ }\left( g \right)\text{ }+\text{ }3H2\text{ }\left( g \right)\text{ }\to \text{ }2NH3\text{ }\left( g \right)\text{ };\text{ }rH0=\text{ }\text{ }92.4\text{ }kJ\text{ }mol1\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-d81c6dc91c7c744bc321c09dcfa91bab_l3.png)
What is the standard enthalpy of development of NH3 gas?
Solution: Standard enthalpy of development of a compound is the adjustment of enthalpy that happens during the arrangement of 1 mole of a substance in its standard structure from its constituent...
Enthalpies of development of CO (g), CO2 (g), N2O (g) and N2O4(g) are – 110, – 393, 81 and 9.7 kJ mol–1 individually. Discover the worth of ∆rH for the response: ![Rendered by QuickLaTeX.com \[N2O4\left( g \right)\text{ }+\text{ }3CO\left( g \right)\to \text{ }N2O\left( g \right)\text{ }+\text{ }3\text{ }CO2\left( g \right)\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-84c70293d06a7f15cf0337e2f45f3df8_l3.png)
Solution: ∆rH for a response is characterized as the contrast between ∆fH worth of items and ∆fH worth of reactants.
What do you understand by isoelectronic species? Name a species that will be isoelectronic with each of the following atoms or ions. (i) 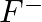 (ii)
(ii) 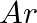 (iii)
(iii) 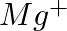 (iv)
(iv) 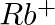
Answer: Isoelectronic species have the same number of electrons as one another. i) The elements $F^-$ and $O_2$ each have ten electrons. As a result, they are considered isoelectronic. (ii) The...
How do atomic radius vary in a period and in a group? How do you explain the variation?
Answer: A period's left to right movement reduces atomic radius. Because external electrons are available in a comparable valence shell, the atomic number increases from left to right, increasing...
Enthalpy of burning of carbon to CO2 is – 393.5 kJ mol–1. Compute the warmth endless supply of 35.2 g of CO2 from carbon and dioxygen gas.
Solution: Arrangement of CO2 from carbon and dioxygen gas can be addressed as: \[\begin{array}{*{35}{l}} \left( 1\text{ }mole\text{ }=\text{ }44\text{ }g \right) \\ ~ \\ Warmth\text{...
What does atomic radius and ionic radius really mean to you?
Answer: The atomic radius is the size of an atom. It measures an atom's size. If an element is a metal, its radius is metallic, and if it is not a metal, its radius is covalent. The metallic radius...
Why do elements in the same group have similar physical and chemical properties?
Answer: The number of valence electrons in any element determines the chemical and physical properties of that element. When elements in the same group of the periodic table have the same number of...
Which element do you think would have been named by (i) Lawrence Berkeley Laboratory (ii) Seaborg’s group?
Answer: (i) Lawrencium (Lr), which has an atomic number of Z=103, and Berkelium (Bk), which has an atomic number of Z=97, are the two elements with the highest atomic numbers. (ii) Seaborgium (Sg),...
What is the atomic number of element keeping in mind both the cases given below; i) Element is in 3rd period of the periodic table. ii) Element is in 17th group of the periodic table.
Answer: The first phase has two elements, while the second period has eight. As a result, element Z = 11 starts the third phase. The third period currently has eight elements. As a result, the 18th...
In terms of period and group where would you locate the element with Z =114?
Answer: The seventh period of the periodic table contains elements with atomic numbers Z = 87–114. The element with Z = 114 is thus available in the seventh period. In the seventh period, the first...
Work out the enthalpy change on freezing of 1.0 mol of water at 10.0°C to ice at – 10.0°C. ![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} fusH\text{ }=\text{ }6.03\text{ }kJ\text{ }mol1\text{ }at\text{ }0{}^\circ C. \\ ~ \\ Cp\left[ H2O\left( l \right) \right]\text{ }=\text{ }75.3\text{ }J\text{ }mol1\text{ }K\text{ }\text{ }1 \\ ~ \\ Cp\left[ H2O\left( s \right) \right]\text{ }=\text{ }36.8\text{ }J\text{ }mol1\text{ }K\text{ }\text{ }1 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ae9ce04526cb31bbc938dd00ac9ef2b9_l3.png)
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} fusH\text{ }=\text{ }6.03\text{ }kJ\text{ }mol1\text{ }at\text{ }0{}^\circ C. \\ ~ \\ Cp\left[ H2O\left( l \right) \right]\text{ }=\text{ }75.3\text{ }J\text{ }mol1\text{ }K\text{ }\text{ }1 \\ ~ \\ Cp\left[ H2O\left( s \right) \right]\text{ }=\text{ }36.8\text{ }J\text{ }mol1\text{ }K\text{ }\text{ }1 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ae9ce04526cb31bbc938dd00ac9ef2b9_l3.png)
Solution: Complete enthalpy change engaged with the change is the amount of the accompanying changes: (a) Energy change engaged with the change of 1 mol of water at 10°C to 1 mol of water at 0°C....
On the basis of quantum numbers, justify that the sixth period of the periodic table should have 32 elements.
Answer: For the outermost shells of a periodic table, a period shows the value of the main quantum number (n). Each period begins with the primary quantum number (n). And n for the 6th period is 6....
What is the basic difference in approach between the Mendeleev’s Periodic Law and the Modern Periodic Law?
Answer: Mendeleev’s Approach for periodic law Modern approach for the periodic law Periodic functions of the atomic mass of the corresponding elements determine the chemical and physical properties...
Which important property did Mendeleev use to classify the elements in his periodic table and did he stick to that?
Answer: Mendeleev arranged the elements in his periodic table by atomic weight. Mendeleev classified the elements into groups and periods based on atomic weight. Mendeleev grouped elements with...
What is the basic theme of organization in the periodic table?
Answer: It divides items into periods and groups based on their qualities. This method simplifies and organizes the study of elements and their compounds. Elements with similar characteristics are...
Work out the quantity of kJ of warmth important to raise the temperature of 60.0 g of aluminum from 35°C to 55°C. Molar warmth limit of Al is 24 J mol–1 K–1
solution: From the statement of warmth (q), \[q\text{ }=\text{ }m.\text{ }c.\text{ }T\] Where, \[\begin{array}{*{35}{l}} c\text{ }=\text{ }molar\text{ }warmth\text{ }limit \\ ~ \\...
Which of the following are the correct reasons for the anomalous behaviour of lithium? (i) The exceptionally small size of its atom (ii) Its high polarising power (iii) It has a high degree of hydration (iv) Exceptionally low ionisation enthalpy
Answer: Option i) & ii) Lithium exhibits a high ionization enthalpy in addition to a high degree of hydration. This is owing to the fact that it is so little.
Choose the correct statements from the following. (i) Beryllium is not readily attacked by acids because of the presence of an oxide film on the surface of the metal. (ii) Beryllium sulphate is readily soluble in water as the greater hydration enthalpy of Be2+ overcomes the lattice enthalpy factor. (iii) Beryllium exhibits coordination number more than four. (iv) Beryllium oxide is purely acidic.
Answer: Option i) & ii) Be mimics Al (diagonal relationship), and together they create a protective film of oxide that is resistant to acid assault. Because of the high hydration enthalpy of...
Identify the correct formula of halides of alkaline earth metals from the following. (i) BaCl2.2H2O (ii) BaCl2.4H2O (iii) CaCl2.6H2O (iv) SrCl2.4H2O
Answer: option i) & iii) The tendency to generate halide hydrates decreases gradually as one moves down the chemical group. The hydrates are MgCl2.6H2O, CaCl2.6H2O, SrCl2.6H2O, and BaCl2.2H2O.
When Zeolite, which is hydrated sodium aluminium silicate is treated with hard water, the sodium ions are exchanged with which of the following ion(s)? (i) 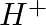 ions (ii)
ions (ii) 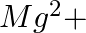 ions (iii)
ions (iii) 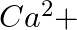 ions (iv)
ions (iv) 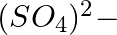 ions
ions
Answer: Option ii) & iii) Because of this, when zeolite, which is sodium aluminium silicate, reacts with hard water, the sodium ion of zeolite is swapped for calcium and magnesium ions.
The response of cyanamide, NH2CN(s), with dioxygen was done in a bomb calorimeter, and ∆U was observed to be – 742.7 kJ mol–1 at 298 K. Ascertain enthalpy change for the response at 298 K. ![Rendered by QuickLaTeX.com \[NH2CN\left( g \right)\text{ }+\text{ }3/2\text{ }O2\left( g \right)\text{ }\to \text{ }N2\left( g \right)\text{ }+\text{ }CO2\left( g \right)\text{ }+\text{ }H2O\left( l \right)\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-6cb518a194b0305964737ad87e3d7289_l3.png)
Solution: Enthalpy change for a response (∆H) is given by the articulation, \[H\text{ }=\text{ }U\text{ }+\text{ }ngRT\] Where, \[\begin{array}{*{35}{l}} U\text{ }=\text{ }change\text{...
Which of the following compounds are readily soluble in water? (i) BeSO4 (ii) MgSO4 (iii) BaSO4 (iv) SrSO4
Answer: Option i) & ii) Because their hydration enthalpies are higher than their lattice enthalpies, BeSO4 and MgSO4 are extremely soluble in water. CaSO4, SrSO4, and BaSO4 have low hydration...
Several sodium compounds find use in industries. Which of the following compounds are used for textile industry? (i) Na2CO3 (ii) NaHCO3 (iii) NaOH (iv) NaCl
Answer: Option i) & iii) Both the chemicals are widely used in industries for preparation of other compounds in large scale.
Metallic elements are described by their standard electrode potential, fusion enthalpy, atomic size, etc. The alkali metals are characterised by which of the following properties? (i) High boiling point (ii) High negative standard electrode potential (iii) High density (iv) Large atomic size
Answer: Option ii) & iv) Periods begin with alkali metals. For their period, alkali metals have the biggest atomic radius. They have low density due to their huge size and low bulk. Alkali...
Dehydration of hydrates of halides of calcium, barium and strontium i.e., CaCl26H2O, BaCl2.2H2O, SrCl2.2H2O, can be achieved by heating. These become wet on keeping in air. Which of the following statements is correct about these halides? (i) act as dehydrating agent (ii) can absorb moisture from the air (iii) The tendency to form hydrate decreases from calcium to barium (iv) All of the above
Answer: Option iv) Because they are hygroscopic in nature, the calcium, barium, and strontium halides operate as a dehydrating agent in the body. They are capable of absorbing moisture....
In a cycle, 701 J of warmth is consumed by a framework and 394 J of work is finished by the framework. What is the adjustment of interior energy for the cycle?
solution: As per the principal law of thermodynamics, \[U\text{ }=\text{ }q\text{ }+\text{ }W\text{ }\left( I \right)\] Where, \[\begin{array}{*{35}{l}} U\text{ }=\text{ }change\text{...
A chemical A is used for the preparation of washing soda to recover ammonia. When CO2 is bubbled through an aqueous solution of A, the solution turns milky. It is used in whitewashing due to disinfectant nature. What is the the chemical formula of A? (i) Ca (HCO3)2 (ii) Cao (iii) Ca(OH)2 (iv) CaCO3
Answer: option iii) Ca(OH)2 is the chemical A that is used in the manufacturing of washing soda Na2CO3 in order to recover ammonia. It is a reaction that occurs during the Solvay process, which is...
Which of the following statements is true about Ca(OH)2? (i) It is used in the preparation of bleaching powder (ii) It is a light blue solid (iii) It does not possess disinfectant property. (iv) It is used in the manufacture of cement.
Answer: Option i) Slaked lime is formed by combining quicklime and water to form a paste. Because quicklime can be used in the manufacture of cement, slaked lime can be used in the manufacture of...
A substance which gives brick red flame and breaks down on heating to give oxygen and a brown gas is (i) Magnesium nitrate (ii) Calcium nitrate (iii) Barium nitrate (iv) Strontium nitrate
Answer: Option ii) The alkali metal compounds and alkaline earth metal compounds give the flame its colour. In a flame, calcium turns brick red, strontium turns crimson red, barium turns apple...
A response ![Rendered by QuickLaTeX.com \[,\text{ }A\text{ }+\text{ }B\text{ }\to \text{ }C\text{ }+\text{ }D\text{ }+\text{ }q\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f134f882cde1f21a1a7d40a02e8eec6e_l3.png)
is found to have a positive entropy change. The response will be (I) conceivable at high temperature (ii) conceivable just at low temperature (iii) unrealistic at any temperature (iv) conceivable at any temperature
Solution: For a response to be unconstrained, ∆G ought to be negative. \[G\text{ }=\text{ }H\text{ }\text{ }TS\] As indicated by the inquiry, for the given response, ...
The formula of soda ash is (i) Na2CO3.10H2O (ii) Na2CO3.2H2O (iii) Na2CO3.H2O (iv) Na2CO3
Answer: Option iv) The formula for soda ash is $Na_2CO_3$ that is sodium carbonate.
Which of the following elements does not form hydride by direct heating with dihydrogen? (i) Be (ii) Mg (iii) Sr (iv) Ba
Answer: Option i) When heated, all of the elements, with the exception of beryllium, mix with hydrogen to create their hydrides.
Suspension of slaked lime in water is known as (i) lime water (ii) quick lime (iii) milk of lime (iv) an aqueous solution of slaked lime
Answer: Option iii) Calcium hydroxide dissociates in an aqueous solution, releasing calcium cations and hydroxide anions. Calcium oxide is the chemical compound known as quicklime. Milk of lime is a...
The enthalpy of burning of methane, graphite and dihydrogen at 298 K are, – 890.3 kJ mol–1 – 393.5 kJ mol–1 , and – 285.8 kJ mol–1 individually. Enthalpy of arrangement of CH4(g) will be (I) – 74.8 kJ mol–1 (ii) – 52.27 kJ mol–1 (iii) +74.8 kJ mol–1 (iv) +52.26 kJ mol–1 .
solution: As indicated by the inquiry, Subsequently, the ideal condition is the one that addresses the development of CH4 (g) i.e., Enthalpy of arrangement of\[~CH4\left( g \right)\text{...
Dead burnt plaster is (i) CaSO4 (ii) CaSO4.1/2 H2O (iii) CaSO4.H2O (iv) CaSO4.2H2O
Answer: Option i) When plaster of Paris is heated to 200°C, it transforms into anhydrous calcium sulphate, often known as dead plaster, which lacks setting properties since it absorbs water at a...
By adding gypsum to cement (i) setting time of cement becomes less. (ii) setting time of cement increases. (iii) colour of cement becomes light. (iv) the shining surface is obtained.
Answer: Option ii) The addition of gypsum serves only to slow down the setting process of the cement, allowing it to solidify to a sufficiently hard state before use.
When sodium is dissolved in liquid ammonia, a solution of deep blue colour is obtained. The colour of the solution is due to (i) ammoniated electron (ii) sodium ion (iii) sodium amide (iv) ammoniated sodium ion
Answer: option i) The concentration of these solvated ions in the sodium in liquid ammonia solution changes the color to copper. So the ammoniated electron is responsible for the solution's color.
In the synthesis of sodium carbonate, the recovery of ammonia is done by treating NH4Cl with Ca(OH)2. The by-product obtained in this process is (i) CaCl2 (ii) NaCl (iii) NaOH (iv) NaHCO3
Answer: Option i) Sodium carbonate is synthesised by Solvary ammonia soda process.
∆Uθof ignition of methane is – X kJ mol–1 . The worth of ∆Hθ is ![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} \left( I \right)\text{ }=\text{ }U\theta \\ ~ \\ \left( ii \right)\text{ }>\text{ }U\theta \\ ~ \\ \left( iii \right)\text{ }<\text{ }U\theta \\ ~ \\ \left( iv \right)\text{ }=\text{ }0 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-253e16b0c605d0c940895437070f698c_l3.png)
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} \left( I \right)\text{ }=\text{ }U\theta \\ ~ \\ \left( ii \right)\text{ }>\text{ }U\theta \\ ~ \\ \left( iii \right)\text{ }<\text{ }U\theta \\ ~ \\ \left( iv \right)\text{ }=\text{ }0 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-253e16b0c605d0c940895437070f698c_l3.png)
Solution: Since \[\begin{array}{*{35}{l}} H\theta \text{ }=\text{ }U\theta \text{ }+\text{ }ngRT\text{ }and\text{ }U\theta \text{ }=\text{ }\text{ }X\text{ }kJ\text{ }mol1\text{ }, \\ ~ \\ H\theta...
Amphoteric hydroxides react with both alkalies and acids. Which of the following Group 2 metal hydroxides is soluble in sodium hydroxide? (i) Be(OH)2 (ii) Mg(OH)2 (iii) Ca(OH)2 (iv) Ba(OH)2
Answer: option i) The solubility of hydroxides of alkaline earth metals in water increases as the alkaline earth metals move from Be to Ba. Both Be(OH)2 and Mg(OH)2 are nearly insoluble in water....
The solubility of metal halides depends on their nature, lattice enthalpy and hydration enthalpy of the individual ions. Amongst fluorides of alkali metals, the lowest solubility of LiF in water is due to (i) Ionic nature of lithium fluoride (ii) High lattice enthalpy (iii) High hydration enthalpy for lithium-ion. (iv) Low ionisation enthalpy of the lithium atom
Answer: Option ii) Lattice and hydration enthalpies encourage the dissolving of alkali metal halides in water. Fluorides are soluble in this order: LiF, NaF, KF, RbF, CsF. The high lattice energy of...
The order of decreasing ionisation enthalpy in alkali metals is (i) Na > Li > K > Rb (ii) Rb < Na < K Na > K > Rb (iv) K < Li < Na < Rb
Answer: option iii) Increasing the size of the atoms in a group results in a decrease in ionization enthalpy. As a result, the following is the ranking: Li > Na > K > Rb.
What happens when: (I) Sodium metal is drenched in water? (ii) Sodium metal is warmed in bounty of air? (iii) Sodium peroxide gets disintegrated in water?
Solution: (I) Sodium responds to shape NaOH and H2 gas when it is dropped in water. The response happens as displayed beneath: \[2Na\left( s \right)\text{ }+\text{ }2H2O\left( l \right)\text{...
Some of the Group 2 metal halides are covalent and soluble in organic solvents. Among the following metal halides, the one which is soluble in ethanol is (i) BeCl2 (ii) MgCl2 (iii) CaCl2 (iv) SrCl2
Answer: Option i) Beryllium haldies are essentially covalent compounds that are soluble in organic solvents such as ethanol and acetic acid.
Metals form basic hydroxides. Which of the following metal hydroxide is the least basic? (i) Mg(OH)2 (ii) Ca(OH)2 (iii) Sr(OH)2 (iv) Ba(OH)2
Answer: Option i) As the ionisation enthalpy increases from Mg to Ba, the M – O bond weakens and weakens down the group, and as a result, basicity increases down the group as a function of time. As...
Which of the carbonates given below is unstable in air and is kept in CO2 atmosphere to avoid decomposition. (i) BeCO3 (ii) MgCO3 (iii) CaCO3 (iv) BaCO3
Answer: Option i) Because of the smaller cation and larger anion sizes (smaller cation stabilises smaller anion through crystal lattice energy), Beryllium Carbonate can only be preserved in CO2...
Metal carbonates decompose on heating to give metal oxide and carbon dioxide. Which of the metal carbonates is most stable thermally? (i) MgCO3 (ii) CaCO3 (iii) SrCO3 (iv) BaCO3
Answer: Option iv) The thermal stability of metal carbonates rises as the metal's electropositive character or basicity increases from Be(OH)2 to Ba(OH)2. The most stable is BaCO3.
Compose adjusted conditions for responses between: (a) Na2O2 and water (b) KO2 and Water (c) Na2O and CO2
Solution: \[\begin{array}{*{35}{l}} ~ \\ \left( a \right)\text{ }2Na2O2\left( s \right)\text{ }+\text{ }2H2O\left( l \right)\text{ }\to \text{ }4NaOH\left( aq \right)\text{ }+\text{ }O2\left( aq...
The reducing power of a metal depends on various factors. Suggest the factor which makes Li, the strongest reducing agent in aqueous solution. (i) Sublimation enthalpy (ii) Ionisation enthalpy (iii) Hydration enthalpy (iv) Electron-gain enthalpy
Answer: option iii) Li's hydration enthalpy is likewise high (highly exothermic). Li atom has the highest hydration enthalpy, making it the strongest reducing agent in aqueous media. - Li atom's...
Alkali metals react with water vigorously to form hydroxides and dihydrogen. Which of the following alkali metals reacts with water least vigorously? (i) Li (ii) Na (iii) K (iv) Cs
Answer: Option i) The answer is Li has a high hydrogen enthalpy. So its water reaction produces a lot of energy, which is used up infusion, vaporization, and ionization. So its water interaction is...
What happens when (I) magnesium is scorched in air (ii) fast lime is warmed with silica (iii) chlorine responds with slaked lime (iv) calcium nitrate is warmed ?
Solution: (iii) When chloride is added to slaked lime, it gives blanching powder. \[Ca\left( OH \right)2\text{ }+\text{ }Cl2\text{ }CaOCl2\text{ }+\text{ }H2O\] Blanching powder (iv)...
The alkali metals are low melting. Which of the following alkali metal is expected to melt if the room temperature rises to 30°C? (i) Na (ii) K (iii) Rb (iv) Cs
Solution: The correct answer is option (iv). The melting point of alkali metals drops as the strength of metallic bonding diminishes with increasing the size of the atom, as shown in the graph. As a...
Starting with sodium chloride how would you proceed to prepare (i) sodium metal (ii) sodium hydroxide (iii) sodium peroxide (iv) sodium carbonate?
Solution: (a) Sodium can be extricated from sodium chloride by Downs measure. This interaction includes the electrolysis of intertwined NaCl (40%) and CaCl2 (60 %) at a temperature of 1123 K in...
Assertion (A): Beryllium carbonate is kept in the atmosphere of carbon dioxide. Reason (R): Beryllium carbonate is unstable and decomposes to give beryllium oxide and carbon dioxide. (i) Both A and R are correct and R is the correct explanation of A. (ii) Both A and R are correct but R is not the correct explanation of A. (iii) Both A and R are not correct. (iv) A is not correct but R is correct.
Answer: Option (i) is correct. Beryllium carbonate is stable and decomposes to give beryllium oxide and carbon dioxide. The concentration of carbon dioxide grows in the right side, causing the...
Assertion (A): The carbonate of lithium decomposes easily on heating to form lithium oxide and CO2. Reason (R): Lithium being very small in size polarises large carbonate ion leading to the formation of more stable Li2O and CO2. (i) Both A and R are correct and R is the correct explanation of A. (ii) Both A and R are correct but R is not the correct explanation of A. (iii) Both A and R are not correct (iv) A is not correct but R is correct.
Answer: Option i) is correct Lithium carbonate readily decomposes into lithium oxide and carbon dioxide. Others do not decompose. $Li _{2} CO _{3} \stackrel{\Delta}{\longrightarrow} Li _{2} O + CO...
Match the elements given in Column I with the colour they impart to the flame has given in Column II.
Column I(i) Cs (ii) Na (iii) K (iv) Ca (v) Sr (vi) Ba Column II(a) Apple green ((b) Violet (c) Brick red (d) Yellow (e) Crimson red (f) Blue Answer: (i) is f (ii) is d (iii) is b (iv) is c...
Match the compounds given in Column I with their uses mentioned in Column II.
Column I(i) CaCO3 (ii) Ca(OH)2 (iii) Cao (iv) CaSO4 Column II(a) Dentistry, ornamental work (b) Manufacture of sodium carbonate from caustic soda (c) Manufacture of high-quality paper (d) Used in...
Look at the solvency and warm dependability of the accompanying mixtures of the salt metals with those of the antacid earth metals. (a) Nitrates (b) Carbonates (c) Sulfates.
Solution: (I) Solubility: Soluble base metal nitrates, carbonates and sulfates have water solvency. At the point when you drop down the gathering of soluble base metals, you will see that the...
Match the elements given in Column I with the properties mentioned in Column II.
Answer: (i) is c (ii) is b (iii) is d (iv) is a,e Explanation: Li: E is the most negative because of the extremely high hydration enthalpy. Na: NaOH is produced from the letter Na (strong base). One...
What is the structure of BeCl2 molecule in gaseous and solid-state?
Answer: The gaseous/vapour state differs from the solid state in several ways. In the solid state, BeCl2 has a polymeric chain structure, which is similar to that of water. In the presence of...
Why do beryllium and magnesium not impart colour to the flame in the flame test?
Answer: Because of the small atomic and ionic sizes of Be and Mg, their electrons are closely attached to the atom. Flames occur as a result of an electron being excited from one of its energy...
Talk about the different responses that happen in the Solvay interaction.
Solution: We realize that the Solvay interaction is utilized in the readiness of sodium carbonate. This interaction is financially savvy contrasted with different cycles of arrangement of Sodium...
Write Lewis structure of 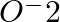 ion and find out oxidation state of each oxygen atom? What is the average oxidation state of oxygen in this ion?
ion and find out oxidation state of each oxygen atom? What is the average oxidation state of oxygen in this ion?
Answer: Because an oxygen atom with zero charges contains six electrons, the oxidation state of the atom is zero. An atom of oxygen with a negative charge contains seven electrons, while when it has...
In the Solvay process, can we obtain sodium carbonate directly by treating the solution containing 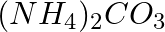 with sodium chloride? Explain.
with sodium chloride? Explain.
Answer: The Solvay method passes $CO_2$ through a concentrated sodium chloride solution containing ammonia, forming ammonium carbonate and ammonium hydrogen carbonate. Crystals of ammonium hydrogen...
All compounds of alkali metals are easily soluble in water but lithium compounds are more soluble in organic solvents. Explain.
Answer: As a result of their large ionic size and low activation enthalpy, alkali metal compounds create ionic compounds, whereas lithium produces covalent compounds as a result of its small ionic...
Why are BeSO4 and MgSO4 readily soluble in water while CaSO4, SrSO4 and BaSO4 are insoluble?
Answer: CaSO4, SrSO4, and BaSO4 are insoluble in water, whereas BeSO4 and MgSO4 are readily soluble in water. This is because to the higher hydration enthalpies of the Be2+ and Mg2+ ions, which...
Discuss the trend of the following: (i) Thermal stability of carbonates of Group 2 elements. (ii) The solubility and the nature of oxides of Group 2 elements.
Answer: i) Carbonate thermal stability rises with cationic size. The more stable an alkaline earth metal's oxide, the less stable its carbonate. As BeO is stable, BeCO3 is not. (ii) Alkali metals...
In what ways lithium shows likenesses to magnesium in its compound conduct?
Solution: Likenesses among lithium and magnesium: (I) lithium and magnesium respond delayed with cold water. (ii) oxides of lithium and magnesium are less dissolvable in H2O. additionally the...
Name an element from Group 2 which forms an amphoteric oxide and a water-soluble sulphate.
Answer: Beryllium is from Group 2. Unlike the other chemicals, beryllium oxide is amphoteric. Group 2 sulfates are water-soluble, as is $BeSO_4$.
Lithium resembles magnesium in some of its properties. Mention two such properties and give reasons for this resemblance.
Answer: i) In terms of weight and hardness, lithium and magnesium are both significantly lighter and tougher than the other metals in their respective families. (ii) Both LiCl and MgCl2 halides are...
Complete the following reactions (i) 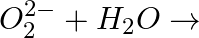 (ii)
(ii) 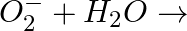
Answer: (i) Peroxide ions react with water and form $H _{2} O _{2}$. $O _{2}^{2-}+2 H _{2} O \longrightarrow 2 OH ^{-}+ H _{2} O _{2}$ (ii) Superoxides react with water and form $H _{2} O _{2}$...
2.9 g of a gas at 95 °C involved a similar volume as 0.184 g of dihydrogen at 17 °C, at a similar tension. What is the molar mass of the gas?.
Solution:
When heated in air, the alkali metals form various oxides. Mention the oxides formed by Li, Na and K.
Answer: The reactivity of alkali metals towards oxygen rises with atomic size. So Li only produces LiO2 (Li2O). Sodium forms mostly sodium peroxide and a little sodium oxide, while potassium forms...
How do you account for the strong reducing power of lithium in aqueous solution?
Answer: Lithium has the largest negative E value of any element, measuring –3.04V. Lithium has tiny atomic size and the highest ionization enthalpy of all the elements, although this is offset by...
What will be the strain applied by a combination of 3.2 g of methane and 4.4 g of carbon dioxide contained in a 9 dm3 jar at 27 °C?
Solution: we know that,
sing the condition of state pV=nRT; show that at a given temperature thickness of a gas is corresponding to gas pressure p.
Solution: The condition of state is given by, \[\begin{array}{*{35}{l}} pV\text{ }=\text{ }nRT\text{ }\ldots \text{ }..\left( 1 \right) \\ ~ \\ Where,\text{ }p\text{ }=\text{...
What are electron lacking mixtures? Are BCl3 and SiCl4 electron insufficient species? Clarify.
solution: Electron-lacking mixtures are substance compounds with inadequate octets that will in general get at least 1 electrons to finish their octet setups. BCl3 BCl3 is a genuine illustration of...
Which one of the basic earth metal carbonates is thermally the most steady? (a) MgCO3 (b) CaCO3 (c) SrCO3 (d) BaCO3
Solution: (d) BaCO3 Warm dependability is straightforwardly corresponding to the size of the cation i.e., bigger the size of the iota, more prominent is its warm security. The greatest cation among...
Which one of the accompanying soluble base metals gives hydrated salts? (a) Li (b) Na (c) K (d) Cs
Solution: (a) Li Li is fit for shaping hydrated salts in light of its size. Since it is more modest in size, it has a higher charge thickness and can undoubtedly draw in water atoms around it and...
Which of the accompanying soluble base metals has the most un-dissolving point? (a) Na (b) K (c) Rb (d) Cs
Solution: (d) Cs Cs has the most un-liquefying point of the given salt metals since it has the biggest size. Because of a bigger size, the limiting capacity of Cs is restricted and the grid energy...
How might you clarify the accompanying perceptions? (I) BeO is practically insoluble however BeSO4 is insoluble in water. (ii) BaO is solvent yet BaSO4 is insoluble in water. (iii) LiI is more solvent than KI in ethanol.
Solution: (I) The measures of Be2+ and O2-are little and are profoundly viable with one another. Because of this, a high measure of cross section energy is delivered during its arrangement. The...
State with regards to why (a) an answer of Na2CO3 basic in nature? (b) salt metals are ready by electrolysis of their combined chlorides? (c) Sodium is observed to be more helpful than potassium?
Solution: (a) Sodium bicarbonate and sodium hydroxide are the finished results when Na2CO3 is hydrolyzed. Since, the item are basic in nature, an answer of Na2CO3 is viewed as basic in nature. (b)...
Remark on every one of the accompanying perceptions: (a) The mobilities of the salt metal particles in fluid arrangement are Li+ < Na+ < K+ < Rb+ < Cs+ (b) Lithium is the main metal to frame a nitride straightforwardly. (c) ![Rendered by QuickLaTeX.com \[E0\text{ }for\text{ }M2+\left( aq \right)\text{ }+\text{ }2e\to \text{ }M\left( s \right)\text{ }\left( where\text{ }M\text{ }=\text{ }Ca,\text{ }Sr\text{ }or\text{ }Ba \right)\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-30816136accb3333fde729011cce90bd_l3.png)
is almost steady.
Solution: (a) The ionic and nuclear sizes of the metals will in general increment while going down the antacid gathering. The expanding request of the ionic sizes of the salt metal particles is as...
Clarify the meaning of sodium, potassium, magnesium and calcium in organic liquids.
Solution: Sodium (Na): They are found in our blood plasma and the interstitial liquids around the cells. They help in (a) Transmission of nerve signals. (b) They direct the progression of water...
For what reason is LiF practically insoluble in water while LiCl dissolvable in water as well as in CH3)2CO?
Solution: LiF has a more noteworthy ionic person than LiCl which upsets the harmony between hydration energy and cross section energy. This equilibrium is critical for the reasonability of particles...
For what reason are lithium salts normally hydrated and those of the other antacid particles typically anhydrous?
Solution: Since Lithium has the littlest size among all the soluble base metals, it can without much of a stretch enraptured water atoms. Subsequently, more modest the size of the particle, more...
Portray the significance of the accompanying : (I) limestone (ii) concrete (iii) mortar of paris.
Solution: Employments of concrete: Extension development Putting Most significant fixing in concrete Employments of Plaster of Paris: Used to make projects and shape Used to make careful wraps...
The hydroxides and carbonates of sodium and potassium are effectively solvent in water while the relating salts of magnesium and calcium are sparingly dissolvable in water. Clarify.
solution: Since the nuclear sizes of magnesium and calcium are more modest than that of sodium and potassium, calcium and magnesium structure carbonates and hydroxides with higher cross section...
Draw the design of (I) BeCl2 (fume) (ii) BeCl2 (strong).
solution: BeCl2 has a straight construction and exists as a monomer in the fume state. 2. In the strong stage, BeCl2 is a polymer.
Depict two significant employments of every one of the accompanying : (I) harsh pop (ii) sodium carbonate (iii) quicklime.
solution: (I) Caustic pop (a) Heavily utilized in cleanser ventures. (b) Common reagent in research centers. (ii) Sodium carbonate (a) Finds utilizes in both cleanser and glass ventures. (b) Also...
For what reason is Li2CO3 deteriorated at a lower temperature while Na2CO3 at higher temperature?
Solution: The electropositive person increments while dropping down in the gathering of soluble base metal which brings about an increment in solidness of antacid carbonates. For the most part,...
Potassium carbonate can’t be ready by Solvay measure. Clarify why?
Solution: Solvay measure isn't pertinent for the arrangement of potassium carbonate since potassium carbonate is dissolvable in water and it doesn't hasten out like sodium bicarbonate.
Beryllium and magnesium don’t offer tone to fire though other basic earth metals do as such. Why?
solution: The valence electrons get eager to a higher energy level when a basic earth metal is warmed. It transmits energy which has a place with the apparent locale when this invigorated electron...
At the point when a soluble base metal breaks up in fluid alkali the arrangement can procure various tones. Clarify the explanations behind this kind of shading change.
solution: At the point when the soluble base metal is broken up in fluid alkali, a dark blue shaded arrangement is framed. \[M+\left( x+y \right)NH3\to M\left( NH3 \right)x+e-{}^\text{1}\text{...
For what reason are potassium and caesium, as opposed to lithium utilized in photoelectric cells?
Solution: Lithium, potassium, and cesium, are all antacid metals. Yet at the same time, potassium and cesium are utilized in photoelectric cell and not Lithium since Li is more modest in size when...
In three moles of ethane (C2H6), calculate the following: (i) Number of moles of carbon atoms. (ii) Number of moles of hydrogen atom (iii) Number of molecules of ethane
(a) 1 mole ${{C}_{2}}{{H}_{6}}$ contains two moles of C- atoms. ∴∴ No. of moles of C- atoms in 3 moles of ${{C}_{2}}{{H}_{6}}$ = 2 * 3 = 6 (b) 1 mole ${{C}_{2}}{{H}_{6}}$ contains six moles of H-...
Calculate the atomic mass (average) of chlorine using the following data:
Average atomic mass of Cl. = $[(\text{Fractional abundance of }\!\!~\!\!\text{ }_{{}}^{35}Cl)(\text{molar mass of }\!\!~\!\!\text{ }_{{}}^{35}Cl)+(\text{fractional abundance of }\!\!~\!\!\text{...
Determine the molecular formula of an oxide of iron, in which the mass percent of iron and oxygen are 69.9 and 30.1, respectively.
Mass percent of Fe = 69.9% Mass percent of O = 30.1% No. of moles of Fe present in oxide =$ \frac{69.90}{55.85}$ = 1.25 No. of moles of O present in oxide =$\frac{30.1}{16.0}$ =1.88 Ratio of Fe to...
How much copper can be obtained from 100 g of copper sulphate (CuSO4)?
1 mole of $CuSO_{4}$ contains 1 mole of Cu. Molar mass of $CuSO_{4}$ = (63.5) + (32.00) + 4(16.00) = 63.5 + 32.00 + 64.00 = 159.5 grams 159.5 grams of $CuSO_{4}$ contains 63.5 grams of Cu....
Calculate the mass of sodium acetate 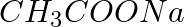 required to make 500 mL of 0.375 molar aqueous solution. Molar mass of sodium acetate is 82.0245 g mol–1.
required to make 500 mL of 0.375 molar aqueous solution. Molar mass of sodium acetate is 82.0245 g mol–1.
0.375 Maqueous solution of $CH_{3}COONa$ = 1000 mL of solution containing 0.375 moles of $CH_{3}COONa$ Therefore, no. of moles of $CH_{3}COONa$ in 500 mL = 0.1875 mole Molar mass of sodium acetate...
Calculate the amount of carbon dioxide that could be produced when (i) 1 mole of carbon is burnt in air. (ii) 1 mole of carbon is burnt in 16 g of dioxygen. (iii) 2 moles of carbon are burnt in 16 g of dioxygen.
(i) 1 mole of carbon is burnt in air. $C+{{O}_{2}}\to C{{O}_{2}}$ 1 mole of carbon reacts with 1 mole of O2 to form one mole of CO2. Amount of $CO_{2}$ produced = 44 g (ii) 1 mole of carbon...
Determine the empirical formula of an oxide of iron, which has 69.9% iron and 30.1% dioxygen by mass.
Percentage of Fe by mass = 69.9% [As said previously] By mass, 30.1 percent of O2 is present. [As said previously] Relative moles of Fe in iron oxide: = (69.9)x(55.85}/55.8569.9 = 1.25 Relative...
Calculate the mass per cent of different elements present in sodium sulphate 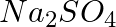
Now for Na2SO4. Molar mass of Na2SO4 = [(2 x 23.0) + (32.066) + 4(16.00)] =142.066 g Therefore, mass percent of the sodium element: = 32.379 = 32.4% Mass percent of the sulphur element: = 22.57...
Calculate the molar mass of the following: (i) 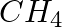 (ii)
(ii)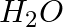 (iii)
(iii)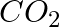
(i)Molecular weight of methane= (1 x Atomic weight of carbon) + (4 x Atomic weight of hydrogen) = [1(12.011 u) +4 (1.008u)] = 12.011u + 4.032 u = 16.043 u (ii)Molecular weight of water= (2 x...
Clarify for what reason can antacid and soluble earth metals not be gotten by synthetic decrease strategies.
Solution: By utilizing a more grounded lessening specialist, the oxides of metals get diminished by the cycle called synthetic decrease. Basic earth metals and soluble base metals are solid among...
Assertion (A): Among isomeric pentanes, 2, 2-dimethylpentane has the highest boiling point. Reason (R): Branching does not affect the boiling point. (i) Both A and R are correct and R is the correct explanation of A. (ii) Both A and R are correct but R is not the correct explanation of A. (iii) Both A and R are not correct. (iv) A is not correct but R is correct.
Option (iii) is correct. Explanation: The lowest boiling point among isomeric pentanes is of 2,2-dimethylpentane, and further on branching, its boiling point decreases
Analyze the salt metals and basic earth metals as for (I) ionization enthalpy (ii) basicity of oxides and (iii) solvency of hydroxides.
Solution: Alkaline earth metals Solubility of hydroxide: They are less soluble compared to alkali metals as it has high lattice energy and is having higher charge densities...
Assertion (A): Nitration of benzene with nitric acid requires the use of concentrated sulphuric acid. Reason (R): The mixture of concentrated sulphuric acid and concentrated nitric acid produces the electrophile, NO2+. (i) Both A and R are correct and R is the correct explanation of A. (ii) Both A and R are correct but R is not the correct explanation of A. (iii) Both A and R are not correct. (iv) A is not correct but R is correct.
Option (i) is correct Explanation: In nitration, benzene is treated with a nitrating mixture, which consists of conc. $HNO_3$ and $H_2SO_4$, and $H_2SO_4$ aids in the production of $NO_2 +$....
Assertion (A): The compound cyclooctane has the following structural formula: It is cyclic and has conjugated 8π-electron system but it is not an aromatic compound.
Reason (R) : (4n + 2) π electrons rule does not hold good and the ring is not planar.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Option (i) is correct. Compounds with the following features are aromatic: planarity and complete delocalization of the electrons in the ring. The ring contains (4n+2) electrons, where n is an...
Match the following reactants in Column I with the corresponding reaction products in Column II.
(i) is d (ii) is c (iii) is b (iv) is a
Match the hydrocarbons in Column I with the boiling points given in Column II.
(i) is b (ii) is c (iii) is a
Match the reagent from Column I which on reaction with CH3 —CH=CH2 gives some product given in Column II as per the codes given below :
(i) is d (ii) is a (iii) is e (iv) is c (v) is b
Suggest a route to prepare ethyl hydrogen sulphate (CH3–CH2–OSO2—OH) starting from ethanol (C2H5OH)
At about 140°C, ethanol is processed with sulphuric acid to produce hydrogen sulphate. The response is
Which of the following compounds are aromatic according to Huckel’s rule?
Huckel's rule states that it must meet the (4n+) rule. Aromatic compounds include B, C, D, and F.
Clarify for what reason is sodium less responsive than potassium.
solution: On dropping down the gathering in the antacid metals, the size of the molecule increments and the impact of the atomic charge gets diminished. Because of these elements, the electron of...
The ring systems having the following characteristics are aromatic. (i) Planar ring containing conjugated π bonds. (ii) Complete delocalisation of the π−electrons in-ring system i.e. each atom in the ring has unhybridised p-orbital, and (iii) Presence of (4n+2)−electrons in the ring where n is an integer (n = 0, 1, 2,………..) [Huckel rule]. Using this information classifies the following compounds as aromatic/non-aromatic.
Aromatic compounds: A, E and F Non-Aromatic : B, C, D and G
Discover the oxidation condition of sodium in Na2O2.
Solution: Leave the oxidation territory of Na alone y. In the event of peroxides, the oxidation condition of oxygen is - 1. Subsequently, \[\begin{array}{*{35}{l}} ~ \\ 2\left( y...
An alkane C8H18 is obtained as the only product on subjecting a primary alkyl halide to Wurtz reaction. On monobromination, this alkane yields a single isomer of a tertiary bromide. Write the structure of alkane and the tertiary bromide.
Write hydrocarbon radicals that can be formed as intermediates during monochlorination of 2-methylpropane? Which of them is more stable? Give reasons.
In comparison to the 1° free radical, the 3° free radical is stabilised by 9 hyperconjugation structures, giving it greater stability.
Write the structures and names of products obtained in the reactions of sodium with a mixture of 1-iodo-2-methylpropane and 2-iodopropane.
When a combination of 1-iodo-2-methylpropane and 2-iodopropane is treated with salt, it produces three compounds as a result of intermolecular and intramolecular reactions:
For what reason are antacid metals not found in nature?
Solution: Sodium, cesium, lithium, francium, potassium, rubidium all together include the salt metals. They comprise of just a single electron on its valence shell, which gets free effectively...
The relative reactivity of 1°, 2°, 3° hydrogen’s towards chlorination is 1: 3.8: 5. Calculate the percentages of all mono-chlorinated products obtained from 2-methyl butane.
Number of hydrogen reactivity = number of mono-chlorinated compounds 1° H = 9 1 = 9 mono-chlorinated products 2° H = 2 3.8 = 7.6 mono-chlorinated products 3° H = 1 5 = 5 mono-chlorinated products...
Nucleophiles and electrophiles are reaction intermediates having electron-rich and electron-deficient centres’ respectively. Hence, they tend to attack electron-deficient and electron-rich centres respectively. Classify the following species as electrophiles and nucleophiles.
Nucleophiles I (vi), (vii), and (viii) Electrophiles are (ii), (iii), (iv), and (v).
Examine the overall qualities and degree in properties of basic earth metals
Solution: General attributes: (I) (Noble gas) ns2 is the electronic design of basic earth metal. (ii) To possess the closest inactive gas setup, these metals lose two of their...
Predict the major product (s) of the following reactions and explain their formation.
Step 1: Peroxide homolysis to produce free radicals Step 2: Bromine free radical formation ∙C6H5 + H-BR → C6H6 + Br Step 3: hydrogen bromide reacts with an alkyl radical.∙
What are the normal physical and synthetic provisions of soluble base metals?
Solution: Actual properties: (1) The soluble base metal is delicate thus we can cut them without any problem. We can ready to cut the sodium metal even by utilizing the blade. (2)...
Suggest a route for the preparation of nitrobenzene starting from acetylene?
I We can cycle acetylene using the intermolecular condensation technique and then treat it at high temperatures in a red hot iron tube. (ii) After being converted to benzene, the aliphatic molecule...
Why does the presence of a nitro group make the benzene ring less reactive in comparison to the unsubstituted benzene ring? Explain.
A nitrogen atom is linked to two extremely electronegative oxygen atoms in the Nitro group. This causes a net reduction in electron density around the nitrogen atom, giving nitrogen a positive charge.
Despite their – I effect, halogens are o- and p-directing in halo-arenes. Explain.
Halogens have a ns2p5 outer configuration, indicating that they may take one electron and are near to completing their octate; halogens have a significant affinity for attracting one electron,...
Arrange the following set of compounds in the order of their decreasing relative reactivity with an electrophile. Give reason.
How will you convert benzene into (i) p – nitrobromobenzene (ii) m – nitrobromobenzene
I Bromine undergoes electrophilic substitution with Br2 in the presence of anhydrous FeBr3 to generate bromobenzene. We get a p-nitrobromobenzene after treating it with conc. HNO3 and conc. H2SO4 at...
What will be the product obtained as a result of the following reaction and why?
Friedel Crafts alkylation with a Lewis acid is demonstrated in this process. The development of the carbocation will occur initially, followed by the production of a more stable secondary...
The intermediate carbocation formed in the reactions of HI, HBr and HCl with propane is the same and the bond energy of HCl, HBr and HI is 430.5 kJ mol-1, 363.7 kJ mol-1 and 296.8 kJ mol-1 respectively. What will be the order of reactivity of these halogen acids?
The ascending order of reactivity is HI >HBr>HCl. The rising order of halogen reactivity corresponds to the rise in bond energy.
Draw Newman and Sawhorse projections for the eclipsed and staggered conformations of ethane. Which of these conformations is more stable and why?
Because there is less C – H bond pair repulsion and the atoms are at their furthest distance from one other, the staggered arrangement is more stable than an eclipse.
Rotation around carbon-carbon single bond of ethane is not completely free. Justify the statement.
In ethane, the single bond is a – bond, which is a coaxial overlap of orbitals that allows the C C bond to rotate on its axis. However, due to the torsional strain that the bond receives as a result...
Alkynes on reduction with sodium in liquid ammonia form trans alkenes. Will the butene thus formed on the reduction of the 2-butyne show the geometrical isomerism?
The negative charge that has generated on one carbon attacks the proton from NH3 and causes another sodium atom to lose its electron, causing the atom to create a second negative charge. The second...
Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain.
To produce a more stable saturated product, alkenes undergo an addition reaction. Hybridization shifts from sp2 to sp3 in this reaction. A substitution process maintains the resonance stability of...
The molecules having dipole moment are __________.(i) 2,2-Dimethylpropane (ii) trans-Pent-2-ene (iii) cis-Hex-3-ene (iv) 2, 2, 3, 3 – Tetramethylbutane.
Option (ii) and (iii) are the answers
Four structures are given in options (i) to (iv). Examine them and select the aromatic structures.
Option (i) and (iii) are the answers.
Which of the following is correct?
Option (i) and (iii) are the answers.
In an electrophilic substitution reaction of nitrobenzene, the presence of nitro group ________. (i) deactivates the ring by an inductive effect. (ii) activates the ring by an inductive effect. (iii) decreases the charge density at ortho and para position of the ring relative to meta position by resonance. (iv) increases the charge density at meta position relative to the ortho and para positions of the ring by resonance
Option (i) and (iii) are the answers.
For an electrophilic substitution reaction, the presence of a halogen atom in the benzene ring _______. (i) deactivates the ring by the inductive effect (ii) deactivates the ring by resonance (iii) increases the charge density at ortho and para position relative to meta position by resonance (iv) directs the incoming electrophile to meta position by increasing the charge density relative to ortho and para position.
The solutions are options (i) and (iii).
Which are the correct IUPAC names of the following compound?;(i) 5 – (2′, 2′–Dimethylpropyl)-decane (ii) 4 – Butyl – 2,2– dimethylnonane (iii) 2,2– Dimethyl – 4– pentyloctane (iv) 5 – neo-Pentyldecane
Option (i) and (iv) are the answers.
Which are the correct IUPAC names of the following compound?;(i) 5– Butyl – 4– isopropyldecane (ii) 5– Ethyl – 4– propyldecane (iii) 5– sec-Butyl – 4– iso-propyldecane (iv) 4–(1-methoxymethyl)– 5 – (1-methyl propyl)-decane
The solutions are options (iii) and (iv).
Which of the following alkenes on ozonolysis give a mixture of ketones only?
The solutions are options (iii) and (iv).
In the following questions, two or more options may be correct. Some oxidation reactions of methane are given below. Which of them is/are controlled oxidation reactions? (i) CH4 (g) + 2O2 (g)→ CO2 (g) + 2H2O (l) (ii) CH4 (g) + O2 (g) → C (s) + 2H2O (l) (iii) CH4 (g) + O2 (g) →(Mo O2 3) HCHO + H2O (iv) 2CH4 (g) + O2 (g) → (Cu/523/100 atm) 2CH3OH
The solutions are options (iii) and (iv).
Which of the following reactions of methane is incomplete combustion:
Option (iii) is the answer.
Arrange the following alkyl halides in decreasing order of the rate of β– elimination reaction with alcoholic KOH.(i) A > B > C (ii) C > B > A (iii) B > C > A (iv) A > C > B
The solution is option (iv).
Arrange the following carbanions in order of their decreasing stability. (A) H3C – C ≡ C– (B) H – C ≡ C– (C) H3C-CH–2 (i) A > B > C (ii) B > A > C (iii) C > B > A (iv) C > A > B
The solution is option (ii).
Arrange the following hydrogen halides in order of their decreasing reactivity with propane. (i) HCl > HBr > HI (ii) HBr > HI > HCl (iii) HI > HBr > HCl (iv) HCl > HI > HBr
The solution is Option (iii)
The enthalpies of all components in their standard states are: (I) Unity (ii) Zero (iii) < 0 (iv) Different for each component
solution: (ii) Zero
For the interaction to happen under adiabatic conditions, the right condition is: ![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} \left( I \right)\text{ }T\text{ }=\text{ }0 \\ ~\left( ii \right)\text{ }p\text{ }=\text{ }0 \\ \left( iii \right)\text{ }q\text{ }=\text{ }0 \\ ~\left( iv \right)\text{ }w\text{ }=\text{ }0 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-68d24071d9398f3df1fc5ee9b1897869_l3.png)
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} \left( I \right)\text{ }T\text{ }=\text{ }0 \\ ~\left( ii \right)\text{ }p\text{ }=\text{ }0 \\ \left( iii \right)\text{ }q\text{ }=\text{ }0 \\ ~\left( iv \right)\text{ }w\text{ }=\text{ }0 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-68d24071d9398f3df1fc5ee9b1897869_l3.png)
solution: \[\left( iii \right)\text{ }q\text{ }=\text{ }0\] Reason: For an adiabatic interaction heat move is zero, for example \[q\text{ }=\text{ }0.\]
Pick the right reply. A thermodynamic state work is an amount (I) used to decide heat changes (ii) whose worth is free of way (iii) used to decide pressure volume work (iv) whose worth relies upon temperature as it were
(ii) An amount which is autonomous of way. solution: Capacities like strain, volume and temperature relies upon the condition of the framework just and not on the way.
Clarify the actual meaning of Van der Waals boundaries?
solution: The actual meaning of 'a': The greatness of intermolecular appealing powers inside gas is addressed by 'a'. The actual meaning of 'b': The volume of a gas atom is addressed by...
Basic temperature for carbon dioxide and methane are 31.1 °C and – 81.9 °C individually. Which of these has more grounded intermolecular powers and why?
solution: In the event that the basic temperature of a gas is higher, it is simpler to condense. That is the intermolecular powers of fascination among the atoms of gas are straightforwardly...
As far as Charles’ law clarify why – 273°C is the most minimal conceivable temperature.
solution: Charles saw that the volume of certain measure of a gas changes by VO /273.15 for every degree rise or fall in temperature.VO being the volume at 0°C.The volume at any temperature t°C...
What might be the SI unit for the amount pV2T 2/n?
solution: The SI unit for pressure, p is Nm–2 . The SI unit for volume, V is m3. The SI unit for temperature, T is K. The SI unit for the quantity of moles, n is mol. Subsequently, the SI unit for...
A combination of dihydrogen and dioxygen at one bar pressure contains 20% by weight of dihydrogen. Compute the halfway tension of dihydrogen.
solution: Let the heaviness of dihydrogen be 20 g. Let the heaviness of dioxygen be 80 g. No. of moles of dihydrogen (nH2), \[\begin{array}{*{35}{l}} ~ \\ =\text{ }20/2 \\ ~ \\...
Work out the volume involved by 8.8 g of CO2 at 31.1°C and 1 bar pressure. ![Rendered by QuickLaTeX.com \[R\text{ }=\text{ }0.083\text{ }bar\text{ }dm3\text{ }K1\text{ }mol1.\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-63d0fbfbaf1b21bdbcb7644d8def9148_l3.png)
solution: \[\begin{array}{*{35}{l}} ~ \\ pVM\text{ }=\text{ }mRT \\ ~ \\ V=\text{ }mRT/Mp \\ \end{array}\] Here, \[\begin{array}{*{35}{l}} m\text{ }=\text{ }8.8\text{ }g \\ ~ \\ R\text{...
Payload is characterized as the contrast between the mass of uprooted air and the mass of the inflatable. Work out the payload when an inflatable of sweep 10 m, mass 100 kg is loaded up with helium at 1.66 bar at 27°C. ![Rendered by QuickLaTeX.com \[~\left( Thickness\text{ }of\text{ }air\text{ }=\text{ }1.2\text{ }kg\text{ }m3\text{ }and\text{ }R\text{ }=\text{ }0.083\text{ }bar\text{ }dm3\text{ }K1\text{ }mol1 \right)\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-106263d412dec0c1dbf32f23a40c938c_l3.png)
solution: Given: \[r\text{ }=\text{ }10\text{ }m\] Accordingly, volume of the inflatable \[\begin{array}{*{35}{l}} =4/3\text{ }\pi r{}^\text{3} \\ ~ \\ =4/3\text{ }\times...
Work out the all out strain in a combination of 8 g of dioxygen and 4 g of dihydrogen restricted in a vessel of 1 dm3 at 27°C. ![Rendered by QuickLaTeX.com \[R\text{ }=\text{ }0.083\text{ }bar\text{ }dm3\text{ }K1\text{ }mol1\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-48f6d7112751bbb16471c7a7ca51ec8d_l3.png)
solution : Given: \[Mass\text{ }of\text{ }O2\text{ }=\text{ }8\text{ }g\]
What amount of time would it require to circulate one Avogadro number of wheat grains, if 1010 grains are appropriated each second?
solution: \[Avogadro\text{ }number\text{ }=\text{ }6.02\text{ }\times \text{ }1023\]Thus, time required
Ascertain the complete number of electrons present in 1.4 g of dinitrogen gas.
solution: \[Molar\text{ }mass\text{ }of\text{ }dinitrogen\text{ }\left( N2 \right)\text{ }=\text{ }28\text{ }g\text{ }mol-1\] Subsequently, 1.4 g of N2 \[\begin{array}{*{35}{l}} 1.4/2.8 \\ ~ \\...
Work out the temperature of 4.0 mol of a gas involving 5 dm3 at 3.32 bar. ![Rendered by QuickLaTeX.com \[\left( R\text{ }=\text{ }0.083\text{ }bar\text{ }dm3\text{ }K1\text{ }mol1 \right).\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0eea6131e45ed685db113cdc3bf5955a_l3.png)
Solution: Given, \[\begin{array}{*{35}{l}} Measure\text{ }of\text{ }the\text{ }gas\text{ },n~=\text{ }4.0\text{ }mol \\ ~ \\ Volume\text{ }of\text{ }the\text{ }gas~,V~=\text{ }5\text{ }dm3 ...
An understudy neglected to add the response blend to the round lined cup at 27 °C however rather he/she set the flagon on the fire. After a pass of time, he understood his error, and utilizing a pyrometer he discovered the temperature of the carafe was 477 °C. What part of air would have been removed out?
solution: Leave the volume of the round lined cup alone V. Then, at that point, the volume of air inside the cup at 27° C is V. Presently, \[\begin{array}{*{35}{l}} ~ \\ V1\text{...
34.05 mL of phosphorus fume weighs 0.0625 g at 546 °C and 0.1 bar pressure. What is the molar mass of phosphorus?
solution: Given, \[\begin{array}{*{35}{l}} p\text{ }=\text{ }0.1\text{ }bar \\ ~ \\ V\text{ }=\text{ }34.05\text{ }mL\text{ }=\text{ }34.05\text{ }\times \text{ }103\text{ }L\text{ }=\text{...
The thickness of a gas is observed to be 5.46 g/dm3 at 27 °C at 2 bar pressure. What will be its thickness at STP?
Solution: For an optimal gas \[Thickness\text{ }\rho \text{ }=\text{ }p\text{ }x\text{ }M/RT\] From the given information, \[\begin{array}{*{35}{l}} 5.46\text{ }=\text{ }2\text{...
What will be the tension of the vaporous blend when 0.5 L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are presented in a 1L vessel at 27°C?
Solution: From the situation \[Pv\text{ }=\text{ }n\text{ }RT\] for the two gases. We can compose \[\begin{array}{*{35}{l}} 0.8\text{ }x\text{ }0.5\text{ }=\text{ }nH2~x\text{ }RT~\text{...
