If the latus rectum of an ellipse is equal to half of minor axis, then find its eccentricity.
Find the equation of a circle concentric with the circle x^2 + y^2 – 6x + 12y + 15 = 0 and has double of its area.
Given equation of the circle is \[{{x}^{2}}~-\text{ }6x\text{ }+\text{ }{{y}^{2}}~+\text{ }12y\text{ }+\text{ }15\text{ }=\text{ }0\] The above equation can be written as \[\begin{array}{*{35}{l}}...
If the line y = √3x + k touches the circle x2 + y2 = 16, then find the value of k.
Find the equation of the circle having (1, –2) as its centre and passing through 3x + y = 14, 2x + 5y = 18
Solving the given equations, \[\begin{array}{*{35}{l}} 3x\text{ }+\text{ }y\text{ }=\text{ }14\text{ }\ldots \ldots \ldots .1 \\ 2x\text{ }+\text{ }5y\text{ }=\text{ }18\text{ }\ldots \ldots \ldots...
Find the equation of a circle which touches both the axes and the line 3x – 4y + 8 = 0 and lies in the third quadrant.
The equation of the given circle is \[{{x}^{2}}~+\text{ }{{y}^{2}}~+\text{ }4x\text{ }+\text{ }4y\text{ }+\text{ }4\text{ }=\text{ }0.\]
If the lines 3x – 4y + 4 = 0 and 6x – 8y – 7 = 0 are tangents to a circle, then find the radius of the circle.
Given lines are 6x – 8y + 8 = 0 and 6x – 8y – 7 = 0. Distance d between two parallel lines y = mx + c1 and y = mx + c2 is given by d = |C1–C2|/√(A2 + B2 ) These parallel lines are tangent to a...
Find the equation of the circle which touches x-axis and whose centre is (1, 2).
Since the circle has a centre (1, 2) and also touches x-axis. Radius of the circle is, r = 2 The equation of a circle having centre (h, k), having radius as r units, is \[{{\left( x\text{ }-\text{...
If a circle passes through the point (0, 0) (a, 0), (0, b) then find the coordinates of its centre.
The equation of a circle having centre (h, k), having radius as r units, is \[{{\left( x\text{ }-\text{ }h \right)}^{2}}~+\text{ }{{\left( y\text{ }-\text{ }k \right)}^{2}}~=\text{ }{{r}^{2}}\]...
Show that the point (x,y) given by x= 2at/1+t^2 and y= a(1-t^2)/1+t^2 lies on a circle for all real values of t such that -1<=t<=1 where a is any given real number
Find the equation of the circle which touches the both axes in first quadrant and whose radius is a.
The circle touches both the x and y axes in the first quadrant and the radius is a. For a circle of radius a, the centre is (a, a). The equation of a circle having centre (h, k), having radius as r...
18 mice were placed in two experimental groups and one control group, with all groups equally large. In how many ways can the mice be placed into three groups?
Solution: As per the question, 18 = No. of mice 3 = No. of groups As the groups are equally large, No. of mice in each group $=6$ mice No. of ways of placement of mice $=18!$ The placement of mice...
The second and third lines of progress components look like each other significantly more than they take after the primary line. Clarify why?
Solution: Because of helpless f orbital safeguarding, powerful atomic charge increments and there is a compression in the size of the third-column components. This compression in size is called...
In spite of the fact that +3 oxidation states are the trademark oxidation condition of lanthanoids cerium shows +4 oxidation state too. Why?
Solution: Ce – [Xe] 4f1 5d1 6s2. For the most part, lanthanoids lose the 5d and 6s electrons and show +3 oxidation state, yet Cerium loses the one 4f electron additionally to achieve Xenon's...
In spite of the fact that Zr has a place with 4d and Hf has a place with 5d progress series yet it is very hard to isolate them. Why?
Solution: Because of the lanthanoid compression helpless f, orbital safeguarding prompts an expansion in powerful atomic charge, which lessens the size of Hf. So the nuclear radii of both Zr and Hf...
The distance of the point of intersection of the lines 2x – 3y + 5 = 0 and 3x + 4y = 0 from the line 5x – 2y = 0 is
SOLUTION: The correct option is option(a) Explanation: Given two lines are \[\begin{array}{*{35}{l}} 2x\text{ }-\text{ }3y\text{ }+\text{ }5\text{ }=\text{ }0~\ldots \text{ }\left( i \right) \\...
Ionization enthalpies of Ce, Pr and Nd are higher than Th, Pa and U. Why?
Solution: Th, Pa and U, 5f electrons begin filling and they have infiltration lower than 4f electrons for Ce, Pr and Nd. Eliminating 4f electrons will be troublesome, so ionization enthalpy for Th,...
In spite of the fact that Cr3+ and Co2+ particles have similar number of unpaired electrons the attractive snapshot of Cr3+ is 3.87 B.M. furthermore, that of Co2+ is 4.87 B.M. Why?
Solution: Cr3+ has an even electron appropriation and will just have turn attractive second commitment though Co2+ has no balanced circulation of electrons so it will have an orbital attractive...
In spite of the fact that fluorine is more electronegative than oxygen, the capacity of oxygen to balance out higher oxidation states surpasses that of fluorine. Why?
Solution: Fluorine has one unpaired electron and structures a solitary bond, while Oxygen has two unpaired electrons and can shape various bonds along these lines balancing out higher oxidation...
Out of Cu2Cl2 and CuCl2, which is more steady and why?
Solution: CuCl2 is more steady in light of the fact that Cu2+ has a higher electron thickness than Cu+. Cu2+ is more modest in size, has higher powerful atomic charge and subsequently a higher...
A convex polygon has 44 diagonals. Find the number of its sides. [Hint: Polygon of 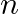 sides has
sides has 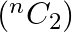
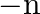 ) number of diagonals.]
) number of diagonals.]
Solution: It is known that, $\begin{array}{l} { }^{n} C_{r} \\ =\frac{n !}{r !(n-r) !} \end{array}$ Let's suppose the no. of sides the given polygon have $=\mathrm{n}$ So now, The no. of line...
Change components show high dissolving focuses. Why?
Solution: Change components have solid metallic bonds. Breaking those bonds becomes more diligently that implies dissolving these components will be troublesome. Thus, liquefying focuses are higher...
The tangent of angles between the lines whose intercepts on the axes are a,-b and b, -a, respectively is: (A) a^2-b^2/ab (B) b^2-a^2/2 (C) b^2-a^2/2ab (D) none of these
The correct option is option(C)-
In an examination, a student has to answer 4 questions out of 5 questions; questions 1 and 2 are however compulsory. Determine the number of ways in which the student can make the choice.
Solution: It is known that, $=\frac{{ }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}}}{\mathrm{r} !(\mathrm{n}-\mathrm{r}) !}$ As per the question, 5 = Total no. of questions 4 = No. of questions to be...
Why E° esteems for Mn, Ni and Zn are more negative than anticipated?
Solution: Mn2+(3d5) and Zn2+(3d10) have half-filled and filled d orbitals which give them dependability and accordingly like to remain as such and not get diminished. With respect to Ni2+(3d8), it...
For what reason does copper not supplant hydrogen from acids?
Solution: A positive decrease potential means the diminished type of Cu is more steady than hydrogen. Consequently, Cu is less receptive than hydrogen and can't dislodge it from acids.
The equation of the straight line passing through the point (3, 2) and perpendicular to the line y = x is A. x – y = 5 B. x + y = 5 C. x + y = 1 D. x – y = 1
The correct option is option(B)- x + y = 5 straight line passing through the point (3, 2) And perpendicular to the line y = x Let the equation of line L be \[y\text{ }-\text{ }{{y}_{1}}~=\text{...
Between sodium hydrogen carbonate and magnesium hydroxide which is a superior acid neutralizer and why?
Solution: Magnesium hydroxide is a superior acid neutralizer in light of the fact that it keeps a pH level in the stomach and insoluble in the stomach. It doesn't make stomach antacid though sodium...
What is the essential contrast among germ-killers and sanitizers?
Solution: 1. Germicides are applied to living tissues though sanitizers are applied on non-living substances 2. Germicides are antimicrobial though sanitizers are against microbial as well 3....
What is the logical clarification for the sensation of sorrow?
Solution: A chemical called Noradrenaline controls the emotional episodes. Sorrow can be caused because of the low degree of noradrenaline which hampers the sign exercises in the cerebrum.
What are analgesics?
Solution: The analgesics drug is a neurologically dynamic medication which is utilized to diminish torment. They don't have any incidental effects.
Slope of a line which cuts off intercepts of equal lengths on the axes is A. – 1 B. – 0 C. 2 D. √3
The correct option is option(A)- – 1
For what reason is it more secure to utilize cleanser according to the ecological perspective?
Solution: When contrasted with the cleansers are more secure to utilize which is biodegradable. It doesn't have a contaminating nature.
A line cutting off intercept – 3 from the y-axis and the tangent at angle to the x-axis is 3/5, its equation is
A. 5y – 3x + 15 = 0 B. 3y – 5x + 15 = 0 C. 5y – 3x – 15 = 0 D. None of these SOLUTION: The correct option is option(A)- 5y – 3x + 15 = 0
How does the expanding of the hydrocarbon chain of manufactured cleansers influence their biodegradability?
Solution: Lesser the expanding lesser is the non-dirtying nature of the cleanser and expanded in fanning causes the contaminating idea of the cleanser to increment.
Draw the outline showing micelle development by the accompanying cleanser. CH3(CH2)10CH2OSO3-Na+
Solution: Micelle formation of the detergent can be shown as:
If p is the length of the perpendicular from origin on the line x/a=y/b=1 and a2,p2,and b2 ae in AP.Then show that a4+b4=0.
Solution: \[\begin{array}{*{35}{l}} \Rightarrow ~\left( {{a}^{2}}~+\text{ }{{b}^{2}} \right)\text{ }\left( {{a}^{2}}~+\text{ }{{b}^{2}} \right)\text{ }=\text{ }2\left( {{a}^{2}}{{b}^{2}} \right) \\...
Dishwashing cleansers are engineered cleansers. What is their compound nature?
Solution: Cleansers are manufactured cleansers which contain non-ionic cleansers that have a purging property. They consolidate with the soil and debasements and make them dissolvable.
Hair shampoos have a place with which class of manufactured cleanser?
Solution: Cationic cleansers are utilized in hair shampoos. eg: cetyltrimethylammonium bromide. Cationic cleansers are quarternary ammonium salts of acetic acid derivations, chlorides or bromides.
Clarify why a few times frothing is found in waterway water close to where sewage water is poured after treatment?
Solution: The froth is because of the non-biodegradable cleansers which are available in water after sewage treatment. A cleanser is a water-solvent purifying specialist which joins with...
In the event that the cleanser has high antacid substance it disturbs the skin. How could the measure of abundance soluble still up in the air? What can be the wellspring of overabundance antacid?
Solution: Overabundance of antacid can be discovered utilizing corrosive base titration. The salt that is shaped during the hydrolyses of oil during cleanser readiness might be a reason for...
What is a delicate cleanser?
Solution: They are effectively solvent which contains potassium salts of unsaturated fats as a significant part.
The two acid neutralizers and antiallergic drugs are antihistamines yet they can’t supplant one another. Clarify why?
Solution: Stomach settling agents are utilized for the treatment of corrosive in the stomach and antihistamines repress the activity of histamine in the body. The two stomach settling agents and...
Anti-inflamatory medicine is an aggravation assuaging antipyretic medication yet can be utilized to forestall coronary failure. Clarify.
Solution: Ibuprofen forestalls blood thickening in the heart as it has against blood-coagulating activity. This activity helps in forestalling coronary failure.
Which class of medications is utilized in dozing pills?
Solution: It contains sedatives as a medication which is intended for the treatment of dread, uneasiness and mental interruptions.
What is the shared trait between the anti-toxin arsphenamine and azodye?
Solution: The kind of linkage moved by the anti-microbial arsphenamine is like that of azodye. anti-toxin arsphenamine gangs – As=As-linkage which is like – N=N-linkage in azodye.
What sort of powers are associated with restricting of substrate to the dynamic site of a catalyst?
Solution: I) Van der Waal power ii) hydrogen holding iii) dipole cooperations iv) ionic securities and so forth are engaged with the limiting of substrate.
Which site of a protein is called allosteric site?
Solution: This is the site other than the dynamic site in which the medications can tie and cause its activity. They control substance responses happening in the human body.
What is the destructive impact of hyperacidity?
Solution: Hyperacidity can cause a ulcer or gastric refluxes in the stomach. The fundamental driver of hyperacidity is the discharge of corrosive in an overabundance sum.
Where are receptors found?
Solution: Receptors are found pon the cell surface layer or inside the cytoplasm. They are organic transducers.
Which sort of medications goes under antimicrobial medications?
Solution: Mostly the antimicrobial medications are utilized to treat the microbial capacities. Models like germicides, sulpha medications and anti-toxins go under this class.
What are germ-killers?
Solution: Germicides are those which are applied to the living body to forestall the development of microorganisms. It is utilized on account of cuts or wounds.
Compose the employments of medications.
Solution: Medications play a significant part in our everyday life. It fixes sicknesses. There are different sorts of medications present as tablets, syrups, salves and so on It advances and keeps...
What is the normal atomic mass of medications?
Solution: Medications have a normal atomic mass of 100-500u.
Which of the accompanying assertions are right? (a) Cationic cleansers have germicidal properties. (b) Bacteria can corrupt the cleansers containing profoundly spread chains. (c) Some engineered cleansers can give froth even in super cold water. (d) Synthetic cleansers are not cleansers.
Solution: (a, c, d) (a) Cationic cleansers are quaternary ammonium salts of amines with acetic acid derivations, chlorides or bromides as anions. These cleansers have germicidal properties. (b)...
Which of coming up next are anionic cleansers? (a) Sodium salts of sulphonated long chain liquor. (b) Ester of stearic corrosive and polyethylene glycol. (c) Quaternary ammonium salt of amine with acetic acid derivation particle. (d) Sodium salts of sulphonated long chain hydrocarbons.
Solution: (a, d) Sodium salts of sulphonated long chain liquor and sodium salts of sulphonated long chain hydrocarbons are anionic cleansers e.g., Sodium laurylsulphate CH3(CH2)10CH2OSO3–Na+ and...
Veronal and Luminal are subsidiaries of barbituric corrosive which are (i) Tranquillizers (ii) Non-narcotic analgesic (iii) Antiallergic drugs (iv) Neurologically active drugs
Solution: (a, d) Tranquilizers are neurologically dynamic medications. Veronal and luminal are subordinates of barbituric corrosive utilized as sedatives.
Amongst the accompanying antihistamine ,which are insect acids? (a)Ranitidine (b) Brompheniramine (c)Terfenadine (d)Cimetidine
Solution: (a, d) Ranitidine and cimetidine are antihistamines which are utilized as subterranean insect acids. These medication bring about arrival of lesser measure of corrosive. Brompheniramine...
Which of the accompanying mixtures are controlled as subterranean insect acids? (a) Sodium carbonate (b)Sodium Hydrogen carbonate (c)Aluminium carbonate (d)Magnism Hydroxide
Solution: (b,d) Sodium Hydrogen carbonate and Magnism Hydroxide ,both are gentle alkalies ,are utilized as subterranean insect acids.
Which of the accompanying assertions are inaccurate with regards to penicillin? (a) An antibacterial organism. (b) Ampicillin is its engineered adjustment. (c) It has bacteriostatic impact. (d) It is a wide range anti-infection.
Solution: (c, d) Penicillin annihilates microbes by obliterating the cell mass of the microorganism or kill the microscopic organisms along these lines, it has bacteriocidal impact. Penicillin has a...
Which of coming up next are antidepressants? (a) Iproniazid (b) Phenelzine (c) Equanil (d) Salvarsan
Solution: (a, b, c) (a) Iproniazid is a hydrazine drug utilized as an upper. (b) Phenelzine is otherwise called Nardil. It is utilized in the treatment of significant burdensome problem. (c) Equanil...
Which of coming up next are sulpha drugs? (a) Sulphapyridine (b) Prontosil (c) Salvarsan (d) Nardil
Solution: (a, b) (a) Sulphapyridine is a sulphonamide antibacterial medication. (b) Prontosil is additionally called sulphamidochrysoidine. (c) Salvarsan is arsenic based antibacterial medication....
Which of the accompanying assertions are right with regards to barbiturates? (a) Hypnotics or rest creating specialists. (b) These are sedatives. (c) Non-opiate analgesics. (d) Pain diminishing without upsetting the sensory system.
Solution: (a, b) Barbiturates are sedatives which are utilized as hypnotics or rest instigating specialists.
Mixtures with clean properties are (a) CHCl, (b) CHI3 (c) Boric corrosive (d) 0.3 ppm watery arrangement of Cl2
Solution: (b, c) (a) CHCl3 (chloroform) was utilized as a sedation in medical procedure yet presently it is utilized in the creation of the Freon refrigerant R-22. (b) Iodoform (CFH3) produces...
Which of coming up next are not utilized as food additives? (a) Table salt (b) Sodium hydrogen carbonate (c) Cane sugar (d) Benzoic corrosive
Solution: (b, d) Table salt and raw sweetener are utilized, as food additives while sodium hydrogen carbonate and benzoic corrosive are not utilized as food additives.
Which of the accompanying assertions are erroneous with regards to receptor proteins? (a) Majority of receptor proteins are installed in the cell layers. (b) The dynamic site of receptor proteins opens within locale of the cell. (c) Chemical couriers are gotten at the limiting locales of receptor proteins. (d) Shape of receptor doesn’t change during connection of courier.
Solution: (b, d) Receptor proteins are implanted in the cell film and their dynamic destinations project outside area of the cell layer. State of the receptor changes during the connection of...
Which of the accompanying won’t improve dietary benefit of food? (a) Minerals (b) Artificial sugars (c) Vitamins (d) Amino acids
Solution: (b) Artificial sugars are non-caloric substitutes for sugar. They are frequently strongly more sweet than sugar however don't upgrade healthy benefit of food. Nutrients and minerals are...
Which of the accompanying synthetic can be added for improving of food things at cooking temperature and doesn’t give calories? (a) Sucrose (b) Glucose (c) Aspartame (d) Sucralose
Solution: (d) Sucralose is a fake improving specialist which is multiple times better than sucrose and doesn't give calories.
Which of the accompanying assertion isn’t correct with regards to chemical inhibitors? (a) Inhibit the synergist movement of the chemical. (b) Prevent the limiting of substrate. (c) Generally, a solid covalent bond is shaped between an inhibitor and a compound. (d) Inhibitors can be cutthroat or non-serious.
Solution: (c) Inhibitors are synthetic substances which will in general lessen the action of a specific chemical. For the most part, a powerless bond, for example, H-holding, van der Waals...
Which of coming up next isn’t an objective particle for drug work in body? (a) Carbohydrates (b) Lipids (c) Vitamins (d) Proteins
Solution: (c) Drugs generally associate with biomolecules like starches, lipids, proteins and nucleic acids. These are called drug targets. Nutrients are not an objective atom for drug work in...
Polyethyleneglycols are utilized in the readiness of which kind of cleansers? (a) Cationic cleansers . (b) Anionic cleansers (c) Non-ionic cleansers (d) Soaps
Solution: (c) Polyethyleneglycols are utilized in the planning of non-ionic cleansers.
Which of coming up next is an illustration of fluid dishwashing cleanser?
Solution: (b) Liquid dishwashing cleansers are non-ionic cleansers.
Glycerol is added to cleanser. It capacities (a) as a filler (b) to increment leathering (c) to forestall quick drying (d) to make cleanser granules
Solution: (c) Glycerol is added to shaving cleanser to forestall quick drying while to upgrade the leathering property of cleanser, a gum called rosin is added to them. It structures sodium rosinate...
Which of the accompanying improves washed property of cleanser? (a) Sodium carbonate (b) Sodium rosinate (c) Sodium stearate (d) Trisodium phosphate
Solution: (b) Shaving cleansers contain glycerol to forestall quick drying. A gum called rosin is included these cleansers which structures sodium rosinate which upgrades washed property of...
A gum rosin added to cleanser to make it foam well. Bithional is added to cleansers to bestow germ-free properties to cleanser. Equanil is (a) fake sugar (b) sedative (c) antihistamine (d) antifertility medication
Solution: (b) Equanil is a sedative utilized in controlling sadness and hypertension.
Compound which is added to cleanser to confer disinfectant properties is (a) sodium laurylsulphate (b) sodium dodecylbenzenesulphonate (c) rosin (d) bithional
Solution: (d) All cleansers are made by bubbling fats or oils with reasonable hydroxide. Varieties are made by adding distinctive natural substances. Sodium laurylsulphate and sodium...
A limited range anti-microbial is dynamic against (a) gram positive or gram negative microbes (b) gram negative microscopic organisms as it were (c) single creature or one sickness (d) both gram positive and gram negative microbes
Solution: (a) A tight range anti-microbial is dynamic against gram positive or gram negative microorganisms.
p1 and p2 are points on either of the two lines y-√3 and |x|=2 at a distance of 5units from their point of intersection.Find the coordinates of the foot of perpendicular drawn from p1 and p2 on the bisector of theangle between the given lines.
Since, \[y\text{ }-\text{ }\surd 3\left| x \right|\text{ }=\text{ }2\]If x ≥ 0, then \[y\text{ }-\text{ }\surd 3x\text{ }=\text{ }2\text{ }\ldots ..\text{ }\left( i \right)\] If x < 0,...
Salvarsan is arsenic containing drug which was first utilized for the treatment of (a) syphilis (b) typhoid (c) meningitis (d) loose bowels
Solution: (a) Salvarsan is arsenic containing drug which was first utilized for treatment of syphilis. Syphilis is an intense and ongoing contaminations sickness brought about by the bacterium...
If the sum of the distances of a moving point in a plane from the axes is 1, then find the locus of the point.
SOLUTION: Let the coordinates of point P be (a, b) Since, the sum of the distance from the axes to the point is always 1 \[\begin{array}{*{35}{l}} \therefore ~\left| x \right|\text{ }+\text{ }\left|...
Which of the accompanying assertion is right? (a) Some sedatives work by hindering the compounds which catalyze the debasement of noradrenaline. (b) Tranquilizers are opiate drugs. (c) Tranquilizers are substance intensifies that don’t influence the message move from nerve to receptor. (d) Tranquilizers are substance intensifies that can calm torment and fever.
Solution: (a) Tranquilizers are neurologically dynamic medications. A few sedatives are antidepressants and the capacities by hindering the proteins which catalyze the corruption of noradrenaline....
Which proclamation about ibuprofen isn’t correct? (a) Aspirin has a place with opiate analgesics. (b) It is powerful in diminishing torment. (c) It has antiblood thickening activity. (d) It is a neurologically dynamic medication.
Solution: (a) Aspirin restrains the combination of mixtures known as prostaglandins which invigorate irritation in the tissues and cause torment. Thus, it is viable in easing torment. Ibuprofen has...
Which is the right assertion about anti-conception medication pills? (a) Contain estrogen as it were (b) Contain progesterone as it were (c) Contain a combination of estrogen and progesterone subsidiaries (d) Progesterone upgrades ovulation
Solution: (c) Birth control pills contain a combination of estrogen and progesterone subsidiaries. Both of these are sex chemicals. Progesterone stifles ovulation and estrogen control the feminine...
Which of the accompanying assertion isn’t right? (a) Some germ-killers can be added to cleansers. (b) Dilute arrangements of certain sanitizers can be utilized as germ-free. (c) Disinfectants are antimicrobial medications. (d) Antiseptic prescriptions can be ingested.
Solution: (d) Antiseptics are applied to the living tissues like injuries, cuts and unhealthy skin surfaces. Disinfectant prescriptions, for example, anti-microbials can't be ingested.
. If A = ![Rendered by QuickLaTeX.com \[\left\{ \mathbf{1},\text{ }\mathbf{2},\text{ }\mathbf{3},\text{ }\mathbf{4}\text{ } \right\}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ddb4a1a003ed727977a64339fa0c6859_l3.png)
,define relations on A which have properties of being: (a) reflexive, transitive but not symmetric (b) symmetric but neither reflexive nor transitive (c) reflexive, symmetric and transitive.
According to the question, \[A\text{ }=\text{ }\left\{ 1,\text{ }2,\text{ }3 \right\}\]. (i) Let \[{{R}_{1}}~=\text{ }\left\{ \left( 1,\text{ }1 \right),\text{ }\left( 1,\text{ }2 \right),\text{...
In a certain city, all telephone numbers have six digits, the first two digits always being 41 or 42 or 46 or 62 or 64. How many telephone numbers have all six digits distinct?
Solution: As per the question, All the telephone numbers have 6 digits Provided that, First 2 digits = 41 or 42 or 46 or 62 or 64 As a result, the no. of two digits that the telephone no. begins...
If 20 lines are drawn in a plane such that no two of them are parallel and no three are concurrent, in how many points will they intersect each other?
Solution: Let 0 be the number of intersection point of first line Let 1 be the number of intersection point of 2nd line Let (2+1) be the number of intersection point of 3rd line Let (3+2+1) be the...
Find the number of integers greater than 7000 that can be formed with the digits 3, 5, 7, 8 and 9 where no digits are repeated. [Hint: Besides 4-digit integers greater than 7000, five digit integers are always greater than 7000.]
Solution: As per the question, The digits that can be used $=3,5,7,8,9$ As, no digits can be repeated, No. of integers is ${ }^{5} \mathrm{P}_{5}=5 !=120$ For a 4 digit integer to be greater than...
If 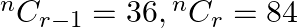 and
and 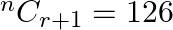 , then find
, then find 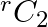 [Hint: From equation using
[Hint: From equation using 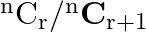 and
and 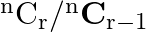 to find the value of r.]
to find the value of r.]
Solution: It is known that, $\begin{array}{l} { }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}} \\ =\frac{\mathrm{n} !}{\mathrm{r} !(\mathrm{n}-\mathrm{r}) !} \end{array}$ As per the question,...
A box contains two white, three black and four red balls. In how many ways can three balls be drawn from the box, if at least one black ball is to be included in the draw.[Hint: Required number of ways 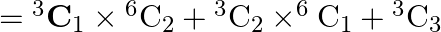 .]
.]
Solution: It is known that, $=\frac{{ }^{n} C_{r}}{r !(n-r) !}$ Now, drawing 1 black and 2 other ball $={ }^{3} \mathrm{C}_{1} \times{ }^{6} \mathrm{C}_{2}$ Now, drawing 2 black and 1 other ball $={...
There are 10 persons named P1,P2,P3, … P10. Out of 10 persons, 5 persons are to be arranged in a line such that in each arrangement P1 must occur whereas P4 and P5 do not occur. Find the number of such possible arrangements. [Hint: Required number of arrangement =7C4× 5!]
Solution: It is known that $\begin{array}{l} { }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}} \\ =\frac{\mathrm{n} !}{\mathrm{r} !(\mathrm{n}-\mathrm{r}) !} \end{array}$ As per the question, There are a...
There are 10 lamps in a hall. Each one of them can be switched on independently. Find the number of ways in which the hall can be illuminated.[Hint: Required number = 210 – 1].
Solution: It is known that, $\begin{array}{l} { }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}} \\ =\frac{\mathrm{n} !}{\mathrm{r} !(\mathrm{n}-\mathrm{r}) !} \end{array}$ It is also known that,...
Two men A and B start with velocities v at the same time from the junction of two roads inclined at 45° to each other. If they travel by different roads, find the rate at which they are being separated.
How about we believe P to be any point where the two streets are leaned at a point of \[45o.\] Presently, two men An and B are moving along the streets PA and PB separately with same speed 'V'....
A kite is moving horizontally at a height of 151.5 meters. If the speed of kite is 10 m/s, how fast is the string being let out; when the kite is 250 m away from the boy who is flying the kite? The height of boy is 1.5 m.
Speed of the kite(V) \[=\text{ }10\text{ }m/s\] Leave FD alone the tallness of the kite and AB be the stature of the kite and AB be the tallness of the kid. Presently, let AF \[=\text{ }x\text{ }m\]...
Solution: (i) 6 alone can fill the Thousand’s Place. As a result, number of way = 1 $$\begin{tabular}{|l|l|l|l|} \hline 6 & & & \\ \hline \end{tabular}$$ Either 0 or 5 can fill the Unit place. As a...
Find the number of different words that can be formed from the letters of the word ‘TRIANGLE’ so that no vowels are together.
Solution: It is known that, $=\frac{{ }^{n} P_{r}}{(n-r) !}$ As per the question, 3 = Total number of vowels letter, 5 = Total number of consonants letter $$\begin{tabular}{|l|l|l|l|l|l|l|l|l|}...
Find the number of permutations of n distinct things taken r together, in which 3 particular things must occur together.
Solution: ${ }^{n} C_{r}$ = Permutations of $n$ distinct things taken $r$ together And when three particular things must occur together, we obtain, $\begin{array}{l} ={ }^{n-3} C_{r-3} \\ ={ }^{n-3}...
A bag contains 5 black and 6 red balls. Determine the number of ways in which 2 black and 3 red balls can be selected from the lot.
Solution: It is known that, $\begin{array}{l} { }^{n} C_{r} \\ =\frac{n !}{r !(n-r) !} \end{array}$ As per the question, 5 = No. of black balls 6 = No. of red balls ${ }^{5} \mathrm{C}_{2}$ = No. of...
How many automobile license plates can be made if each plate contains two different letters followed by three different digits?
Solution: As per the question, 2 = No. of letters in automobile license plates It is known that, There are a total of 26 alphabets Therefore, in the following number of ways, letter can be arranged...
How many committee of five persons with a chairperson can be selected from 12 persons? [Hint: Chairman can be selected in 12 ways and remaining in 11 C4.]
Solution: It is known that, $=\frac{{ }^{n} C_{r}}{r !(n-r) !}$ 12 = No. of ways a chairperson can be selected ${ }^{11} \mathrm{C}_{4}$ = Selection of 4 other people $=\frac{11 !}{4 ! 7 !}=330$...
We wish to select 6 persons from 8, but if the person A is chosen, then B must be chosen. In how many ways can selections be made?
Solution: It is known that, $=\frac{\mathrm{n} !}{\mathrm{r} !(\mathrm{n}-\mathrm{r}) !}$ As per the question, Case 1: If both the persons A and B are selected $=1 \times 1 \times ^{6}...
Out of 18 points in a plane, no three are in the same line except five points which are collinear. Find the number of lines that can be formed joining the point. [Hint: Number of straight lines =18C2 – 5C2 + 1]
Solution: It is known that, $=\frac{\mathrm{n} !}{\mathrm{r} !(\mathrm{n}-\mathrm{r}) !}$ As per question, 18 = Number of points 5 = Number of Collinear points No. of lines made by 18 points $={...
A candidate is required to answer 7 questions out of 12 questions, which are divided into two groups, each containing 6 questions. He is not permitted to attempt more than 5 questions from either group. Find the number of different ways of doing questions.
Solution: It is known that, $\begin{array}{l} { }^{n} C_{r} \\ =\frac{n !}{r !(n-r) !} \end{array}$ 6 = Number of questions in group A 6 = Number of questions in group B As per the question, The...
If the letters of the word RACHIT are arranged in all possible ways as listed in dictionary. Then what is the rank of the word RACHIT? [Hint: In each case number of words beginning with A, C, H, I is 5!]
Solution: As per the question, $$\begin{tabular}{|l|l|l|l|l|l|l|} \hline $\mathrm{R}$ & $\mathrm{A}$ & $\mathrm{C}$ & $\mathrm{H}$ & $\mathrm{I}$ & $\mathrm{T}$ \\ \hline \end{tabular}$$ On...
Eight chairs are numbered 1 to 8. Two women and 3 men wish to occupy one chair each. First the women choose the chairs from amongst the chairs 1 to 4 and then men select from the remaining chairs. Find the total number of possible arrangements.
Solution: It is known that, ${ }^{n} P_{r}$ $=\frac{\mathrm{n} !}{(\mathrm{n}-\mathrm{r}) !}$ As per the question, In 4 different way $W_{1}$ can occupy chairs marked 1 to 4. $$...
The value of 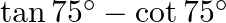 is equal to A.
is equal to A. 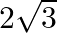 B.
B. 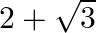 C.
C. 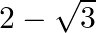 D. 1
D. 1
Solution: Option (A) $2 \sqrt{3}$ is correct. Explanation: As per the question, $\tan 75^{\circ}-\cot 75^{\circ}$ $\begin{array}{l} =\frac{\sin 75^{\circ}}{\cos 75^{\circ}}-\frac{\cos...
If 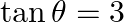 and
and 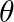 lies in third quadrant, then the value of
lies in third quadrant, then the value of 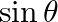 is A.
is A. 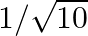 B.
B. 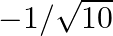 C.
C. 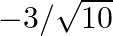 D.
D. 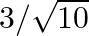
Solution: Option (C) $-3 / \sqrt{10}$ is the correct. Explanation: As per the question, It is given that, $\tan \theta=3$ and $\theta$ lies in the third quadrant $\Rightarrow \cot \theta=1 / 3$ It...
The value of 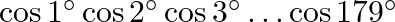 is A.
is A. 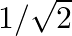 B.
B. 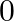 C.
C. 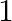 D.
D. 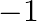
Solution: Option (B) 0 is correct. Explanation: As per the question, As $\cos 90^{\circ}=0$ We have, $\Rightarrow \cos 1^{\circ} \cos 2^{\circ} \cos 3^{\circ} \ldots \cos 90^{\circ} \ldots \cos...
The value of 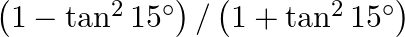 is A.
is A. 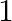 B.
B. 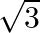 C.
C. 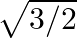 D.
D. 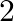
Solution: Option (C) $\sqrt{3 / 2}$ is correct. Explanation: As per the question, Let's say $\theta=15^{\circ} \Rightarrow 2 \theta=30^{\circ}$ Now, as it is known that, $\begin{array}{l}...
The value of 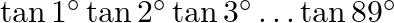 is A. 0 B. 1 C.
is A. 0 B. 1 C. 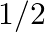 D.
D. 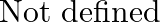
Solution: Option (B) 1 is correct. Explanation: As per the question, $\tan 1^{\circ} \tan 2^{\circ} \tan 3^{\circ} \ldots \tan 89^{\circ}$ $=\tan 1^{\circ} \tan 2^{\circ} \ldots \tan 45^{\circ} \tan...
Which of the following is not correct? A. 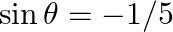 B.
B. 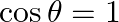 C.
C. 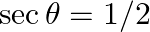 D.
D. 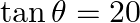
Solution: Option (C) $\sec \theta=1 / 2$ Explanation: As per the question, It is known that, a) $\sin \theta=-1 / 5$ is correct since $\operatorname{Sin} \theta \in[-1,1]$ b) $\cos \theta=1$ is...
If 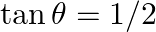 and tan
and tan 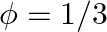 , then the value of
, then the value of 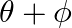 is A.
is A. 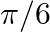 B.
B. 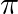 C.
C. 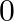 D.
D. 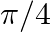
Solution: Option (D) $\pi / 4$ is correct. Explanation: As per the question, $\tan \theta=\frac{1}{2}$ and $\tan \phi=\frac{1}{3}$ It is known that, $\begin{array}{l} \tan (\theta+\phi)=\frac{\tan...
If 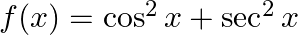 , then A.
, then A. 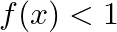 B.
B. 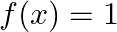 C.
C. 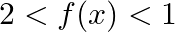 D.
D. 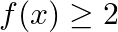 [Hint: A.M ≥ G.M.]
[Hint: A.M ≥ G.M.]
Solution: Option (D) $f(x) \geq 2$ is correcct. Explanation: As per the question, $f(x)=\cos ^{2} x+\sec ^{2} x$ It is known that, A.M $\geq$ G.M. $\begin{array}{l} \Rightarrow \frac{\cos ^{2}...
If 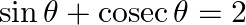 , then
, then 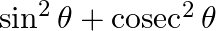 is equal to A. 1 B.4 C. 2 D. None of these
is equal to A. 1 B.4 C. 2 D. None of these
Solution: Option (C) is the correct option. Explanation: As per the question, $\sin \theta+\operatorname{cosec} \theta=2$ Now square both the sides L.H.S. and R.H.S., We have, $\begin{array}{l}...
Find the general solution of the equation 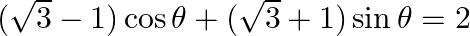 [Hint: Put
[Hint: Put 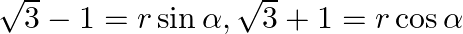 which gives
which gives ![Rendered by QuickLaTeX.com \tan \alpha=\tan ((\pi / 4)-(\pi / 6)) a=\pi / 12]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-6d706c51601ee424f54246630dca1789_l3.png)
Solution: Let's say, $r$ sina $=\sqrt{3}-1$ and $r \cos a=\sqrt{3}+1$ So, $\left.r=\sqrt{(}(\sqrt{3}-1)^{2}+(\sqrt{3}+1)^{2}\right\}=\sqrt{8}=2 \sqrt{2}$ And, tan $\alpha=(\sqrt{3}-1) /(\sqrt{3}+1)$...
Find the general solution of the equation 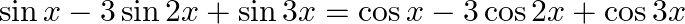
Solution: As per the question, $\sin x-3 \sin 2 x+\sin 3 x=\cos x-3 \cos 2 x+\cos 3 x$ Now grouping $\sin x$ and $\sin 3 x$ in Left Hand Side and, $\cos x$ and $\cos 3 x$ in Right Hand Side, We...
Find the general solution of the equation 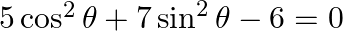
Solution: As per the question, $5 \cos ^{2} \theta+7 \sin ^{2} \theta-6=0$ It is known that, $\sin ^{2} \theta=1-\cos ^{2} \theta$ So, $5 \cos ^{2} \theta+7\left(1-\cos ^{2} \theta\right)-6=0$...
Find the value of the expression 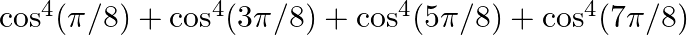 [Hint: Simplify the expression to
[Hint: Simplify the expression to ![Rendered by QuickLaTeX.com 2\left(\cos ^{4} \frac{\pi}{8}+\cos ^{4} \frac{3 \pi}{8}\right)=2\left[\left(\cos ^{2} \frac{\pi}{8}+\cos ^{2} \frac{3 \pi}{8}\right)^{2}-2 \cos ^{2} \frac{\pi}{8} \cos ^{2} \frac{3 \pi}{8}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-fa81990c37138586aab5508ac168ede1_l3.png)
Solution: As per the question, Let's say $y=\cos ^{4}(\pi / 8)+\cos ^{4}(3 \pi / 8)+\cos ^{4}(5 \pi / 8)+\cos ^{4}(7 \pi / 8)$ $\Rightarrow y=\cos ^{4}(\pi / 8)+\cos ^{4}(3 \pi / 8)+\cos ^{4}(\pi-3...
Let f(x) = √x and g (x) = x be two functions defined in the domain R+∪ {0}. Find (i) (fg) (x) (ii) (f/g) (x)
Solution: (i) $(\mathrm{fg})(\mathrm{x})$ $\Rightarrow(f g)(x)=f(x) g(x)$ $\Rightarrow(f g)(x)=(\sqrt{x})(x)$ $\Rightarrow f(x) g(x)=x \sqrt{x}$ (ii) $(f / q)(x)=f(x) / g(x)$...
Let f(x) = √x and g (x) = x be two functions defined in the domain R+∪ {0}. Find (i) (f + g) (x) (ii) (f – g) (x)
Solution: (i) $(f+g)(x)$ $\Rightarrow(f+g)(x)=f(x)+g(x)$ $\Rightarrow f(x)+g(x)=\sqrt{x}+x$ (ii) $(f-g)(x)$ $\Rightarrow(f-g)(x)=f(x)-g(x)$ $\Rightarrow f(x)-g(x)=\sqrt{x}-x$
If 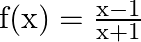 , then show that: (i)
, then show that: (i) 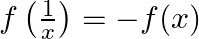 (ii)
(ii) 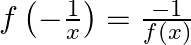
Solution: (i) $f(x)=\frac{x-1}{x+1}$ Now, substitute $\mathrm{x}$ by $1 / \mathrm{x}$, we have $\begin{array}{l} f\left(\frac{1}{x}\right)=\frac{\frac{1}{x}-1}{\frac{1}{x}+1} \\...
Redefine the function f(x) = |x – 2| + |2 + x|, – 3 ≤ x ≤ 3
Solution: As per the question, function $f(x)=|x-2|+|2+x|,-3 \leq x \leq 3$ It is known that, when $x>0$, $|x-2|$ is $(x-2), x \geq 2$ $|2+x|$ is $(2+x), x \geq-2$ when $x>0$ $|x-2|$ is...
Find the range of the following functions given by (i) 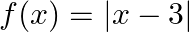 (ii)
(ii) 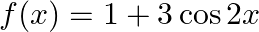
Solution: (i) $f(x)=|x-3|$ As per the question, It is known $|\mathrm{x}|$ are defined for all real values. And $|\mathrm{x}-3|$ will always be greater than or equal to 0 . i.e., $f(x) \geq 0$ As a...
Find the range of the following functions given by (i) 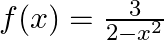 (ii)
(ii) 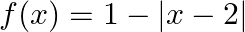
Solution: (i) $f(x)=\frac{3}{2-x^{2}}$ As per the question, Let $\mathrm{f}(\mathrm{x})=\mathrm{y}$, $\begin{array}{l} y=\frac{3}{2-x^{2}} \\ \Rightarrow 2-x^{2}=\frac{3}{y} \\ \Rightarrow...
Find the domain of each of the following functions given by (i) 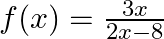
Solution: (i) $f(x)=\frac{3 x}{2 x-8}$ As per the question, For real value of $\begin{array}{l} 28-x \neq 0 \\ \Rightarrow \mathrm{x} \neq 28 \end{array}$ As a result, the domain of...
Find the domain of each of the following functions given by (i) 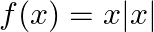 (ii)
(ii) 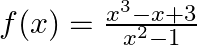
Solution: (i) $f(x)=x|x|$ As per the question, It is known that $x$ and $|x|$ are defined for all real values. As a result, the domain of $f=R$ (ii) $f(x)=\frac{\left(x^{3}-x+3\right)}{x^{2}-1}$ As...
Find the domain of each of the following functions given by (i) 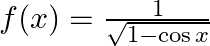 (ii)
(ii) 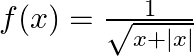
Solution: (i) f(x)=11-cosx \mathrm{f}(\mathrm{x})=\frac{1}{\sqrt{1-\cos \mathrm{x}}} As per the question, It is known that the value of $\cos x$ lies between $-1,1$ $-1 \leq \cos x \leq 1$ Multiply...
Is g = {(1, 1), (2, 3), (3, 5), (4, 7)} a function? Justify. If this is described by the relation, g (x) = αx + β, then what values should be assigned to α and β?
Solution: Provided, $\mathrm{g}=\{(1,1),(2,3),(3,5),(4,7)\}$, and is described by relation $\mathrm{g})$ $(\mathrm{x})=\boldsymbol{\alpha} x+\boldsymbol{\beta}$ Now, given the relation,...
Find the values of x for which the functions f (x) = 3×2 – 1 and g (x) = 3 + x are equal
Solution: Provided, $f$ and $g$ functions defined by $f (x) = {3x}^{2} {-} 1$ and $g (x) = 3 + x$ For what real numbers $x$, $f (x) = g (x)$ In order to satisfy the condition $f(x) = g(x)$, We also...
Express the following functions as set of ordered pairs and determine their range. f: X → R, f (x) = x3 + 1, where X = {–1, 0, 3, 9, 7}
Solution: Provided that, A function $\mathrm{f}: \mathrm{X} \rightarrow \mathrm{R}, \mathrm{f}(\mathrm{x})=\mathrm{x}^{3}+1$, in which $\mathrm{X}=\{-1,0,3,9,7\}$ Domain $= f$ is a function such...
If f and g are two real valued functions defined as f (x) = 2x + 1, g (x) = x2 + 1, then find. (i) fg (ii)f/g
Solution: Provided that, $f$ and $g$ are the real valued functions defined as $f (x) = 2x + 1$, $g (x) = x2 + 1$, (i) $fg$ $\Rightarrow f g=f(x) g(x)$ $=(2 x+1)\left(x^{2}+1\right)$ $=2...
If f and g are two real valued functions defined as f (x) = 2x + 1, g (x) = x2 + 1, then find. (i) f + g (ii) f – g
Solution: Provided that, $f$ and $g$ are the real valued functions defined as $f (x) = 2x + 1$, $g (x) = x^{2} + 1$, (i) $f+g$ $\Rightarrow f+g=f(x)+g(x)$ $=2 x+1+x^{2}+1$ $=x^{2}+2 x+2$ (ii) $f-g$...
Let f and g be real functions defined by f (x) = 2x + 1 and g (x) = 4x – 7. (a) For what real numbers x, f (x) = g (x)? (b) For what real numbers x, f (x) < g (x)?
Solution: Provided that, $f$ and $g$ are the real functions defined by $f(x) = 2x + 1$ and $g(x) = 4x {-} 7$ (a) In order to satisfy the given condition $f(x) = g(x)$, We also need to satisfy, $2x +...
If f and g are real functions defined by f (x) = x2 + 7 and g (x) = 3x + 5, find each of the following (a) (f(t) – f(5))/ (t – 5), if t ≠ 5
Solution: Provided, f and g are real functions such that $f(x)=x^{2}+7$ and $g(x)=3 x+5$ (a) $(f(t)-f(5)) /(t-5)$, if $t \neq 5$ $f(x)=x^{2}+7$ Substitute $x = t$ in $f(x)$, we have $f(t)=t^{2}+7...
If f and g are real functions defined by f (x) = x2 + 7 and g (x) = 3x + 5, find each of the following (a) f (– 2) + g (– 1) (b) f (t) – f (– 2)
Solution: Provided, f and g are real functions such that $f(x)=x^{2}+7$ and $g(x)=3 x+5$ (a) $f(-2)+g(-1)$ $f(x)=x^{2}+7$ Substitute $x = {-}2$ in $f(x)$, we have $f(-2)=(-2)^{2}+7=4+7=11 \ldots...
If f and g are real functions defined by f (x) = x2 + 7 and g (x) = 3x + 5, find each of the following (a) f (3) + g (– 5) (b) f(½) × g(14) (c) f (– 2) + g (– 1)
Solution: Provided, f and g are real functions such that $f(x)=x^{2}+7$ and $g(x)=3 x+5$ (a) $f(3)+g(-5)$ $f(x)=x^{2}+7$ Now substitute $x = 3$ in $f(x)$, we have $f(3)=3^{2}+7=9+7=16 \ldots \dots...
Is the given relation a function? Give reasons for your answer.
Solution: (i) Provided, $t=\{(x, 3) \mid x$ is a real number. As a result, the domain element $x$ is a real number. And also, the range has one number i.e., 3 in it. Hence, in the domain for every...
If 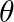 lies in the first quadrant and
lies in the first quadrant and 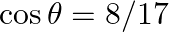 , then find the value of
, then find the value of 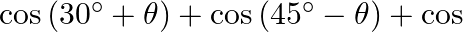
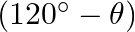
Solution: As per the question, $\begin{array}{l} \cos \theta=8 / 17 \\ \left.\sin \theta=\pm \sqrt{(} 1-\cos ^{2} \theta\right) \end{array}$ As, $\theta$ lies in the first quadrant, so only positive...
If 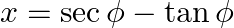 and
and 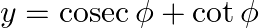 , then show that
, then show that 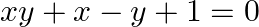 [Hint: Find
[Hint: Find 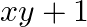 and then show
and then show ![Rendered by QuickLaTeX.com \tan x-y=-(x y+1)]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-e63f068ae68ec5c29ff87464fe3bb668_l3.png)
Solution: As per the question, $x=\sec \phi-\tan \phi$ and $y=\operatorname{cosec} \phi+\cot \phi$ It is given that, $\text {Left Hand Side}=x y+x-y+1$ $\begin{array}{l} =(\sec \phi-\tan...
If 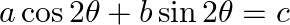 has
has 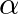 and
and 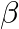 as its roots, then prove that
as its roots, then prove that 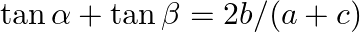 [Hint: Use the identities
[Hint: Use the identities 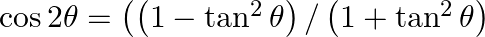 and
and ![Rendered by QuickLaTeX.com \left.\sin 2 \theta=2 \tan \theta /\left(1+\tan ^{2} \theta\right)\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f9cd046638c1b9f63a5af429d92fe81e_l3.png)
Solution: As per the question, $a \cos 2 \theta+b \sin 2 \theta=c$ $\alpha$ and $\beta$ are the roots of the equation. Now using the multiple angles formula, It is known that, $\begin{array}{l} \cos...
Find the value of the expression ![Rendered by QuickLaTeX.com 3\left[\sin ^{4}\left(\frac{3 \pi}{2}-\alpha\right)+\sin ^{4}(3 \pi+\alpha)\right]-2\left[\sin ^{6}\left(\frac{\pi}{2}+\alpha\right)+\sin ^{6}(5 \pi-\alpha)\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0875102a4ed513e4c6f058e4e68399a7_l3.png)
Solution: As per the question, Let's say, $y=3\left[\sin ^{4}(3 \pi / 2-a)+\sin ^{4}(3 \pi+\alpha)\right]-2\left[\sin ^{6}(\pi / 2+\alpha)+\sin ^{6}(5 \pi-\alpha)\right]$ It is known that,...
If cos (θ + ϕ) = m cos (θ – ϕ), then prove that tan θ = ((1 – m)/(1 + m)) cot ϕ [Hint: Express cos (θ + ϕ)/ cos (θ – ϕ) = m/l and apply Componendo and Dividendo]
Solution: As per the question, $\begin{array}{l} \cos (\theta+\phi)=\mathrm{m} \cos (\theta-\phi) \\ \because \cos (\theta+\phi)=\mathrm{m} \cos (\theta-\phi) \\ \Rightarrow \frac{\cos...
If 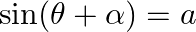 and
and 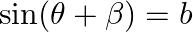 , then prove that
, then prove that 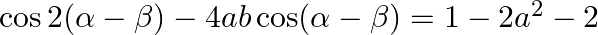
Solution: As per the question, $\sin (\theta+\alpha)=\mathrm{a}$ and $\sin (\theta+\beta)=\mathrm{b}$ $\text {Left Hand Side}=\cos 2(a-\beta)-4 a b \cos (\alpha-\beta)$ Now, using $\cos 2 x=2 \cos...
The inverse square law in electrostatics is 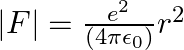 for the force between an electron and a proton. The
for the force between an electron and a proton. The 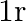 dependence of
dependence of 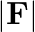 can be understood in quantum theory as being due to the fact that the ‘particle’ of light (photon) is massless. If photons had a mass
can be understood in quantum theory as being due to the fact that the ‘particle’ of light (photon) is massless. If photons had a mass 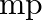 , force would be modified to
, force would be modified to ![Rendered by QuickLaTeX.com |F|=\frac{e^{2}}{\left(4 \pi \epsilon_{0}\right)} r^{2}\left[\frac{1}{r^{2}}+\frac{\lambda}{r}\right] \quad](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-514ad8afbf25cd57a0bc62f92316ce07_l3.png) where
where 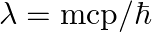 and
and 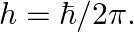 Estimate the change in the ground state energy of an H-atom if
Estimate the change in the ground state energy of an H-atom if 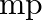 were
were 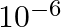 times the mass of an electron.
times the mass of an electron.
$m_{\mathrm{p}} c^{2}=10^{-6} \times$ electron mass $\times c^{2}$ $=10^{-6} \times 0.5 \mathrm{MeV}$ $\approx 10^{-6} \times 0.5 \times 1.6 \times 10^{-13}$ $\approx \mathrm{O} .8 \times 1...
If 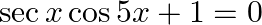 , where
, where 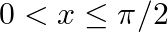 , then find the value of
, then find the value of 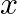
Solution: As per the question, $\sec x \cos 5 x=-1$ $\Rightarrow \cos 5 x=-1 / \sec x$ It is known that, $\sec x=1 / \cos x$ $\Rightarrow \cos 5 x+\cos x=0$ Now by using the transformation formula...
If 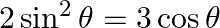 , where
, where 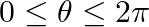 , then find the value of
, then find the value of 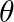 .
.
Solution: As per the question, $2 \sin ^{2} \theta=3 \cos \theta$ It is known that, $\sin ^{2} \theta=1-\cos ^{2} \theta$ Provided that, $2 \sin ^{2} \theta=3 \cos \theta$ $2-2 \cos ^{2} \theta=3...
If 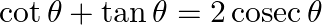 , then find the general value of
, then find the general value of 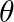 .
.
Solution: As per the question, $\Rightarrow \frac{\cos \theta}{\sin \theta}+\frac{\sin \theta}{\cos \theta}=2 \operatorname{cosec} \theta$ As, $\sin ^{2} \theta+\cos ^{2} \theta=1$ $\Rightarrow...
Find the most general value of 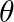 satisfying the equation
satisfying the equation 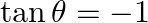 and
and 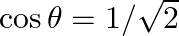
Solution: As per the question, We get, $\tan \theta=-1$ And $\cos \theta=1 / \sqrt{2}$ $\Rightarrow \theta=-\pi / 4$ Therefore, it is known that, $\theta$ lies in IV quadrant. $\theta=2 \pi-\pi /...
The first four spectral lines in the Lyman series of an H-atom are 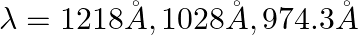 and
and 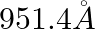 . If instead of Hydrogen, we consider Deuterium, calculate the shift in the wavelength of these lines.
. If instead of Hydrogen, we consider Deuterium, calculate the shift in the wavelength of these lines.
Let the reduced masses of electrons of hydrogen and deuteriur be $\mu _{H}$ and $\mu _{D}$ The fixed mass series for hydrogen and deuterium be $n_i$ and $n_f$ $R_h / R_d=\mu _H / \mu _D$ Reduced...
If 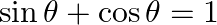 , then find the general value of
, then find the general value of 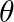 .
.
Solution: As per the question, $\sin \theta+\cos \theta=1$ Since, $\sin \theta+\cos \theta=1$ $\Rightarrow \sqrt{2}\left(\frac{1}{\sqrt{2}} \sin \theta+\frac{1}{\sqrt{2}} \cos \theta\right)=1$ It is...
Find the equations of the lines through the point of intersection of the lines x – y + 1 = 0 and 2x – 3y + 5 = 0 and whose distance from the point (3, 2) is 7/5.
Squaring both the sides, we get
Find the equation of the line which passes through the point (– 4, 3) and the portion of the line intercepted between the axes is divided internally in the ratio 5 : 3 by this point.
On cross multiplication we get \[\begin{array}{*{35}{l}} \Rightarrow ~-72x\text{ }+\text{ }160y\text{ }=\text{ }768 \\ \Rightarrow ~-36x\text{ }+\text{ }80y\text{ }=\text{ }384 \\ \Rightarrow...
If 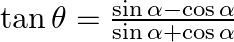 , then show that
, then show that 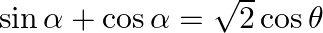 [Hint: Express
[Hint: Express ![Rendered by QuickLaTeX.com \tan \theta=\tan (\alpha-\pi / 2) \theta=\alpha-\pi / 4]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-1f53dae765f177bb1cdf4e56be3c58f7_l3.png)
Solution: It is known that, $\tan \theta=\frac{\sin \alpha-\cos \alpha}{\sin \alpha+\cos \alpha}$ $\Rightarrow \tan \theta=\frac{\cos \alpha\left(\frac{\sin \alpha}{\cos \alpha}-1\right)}{\cos...
A straight line moves so that the sum of the reciprocals of its intercepts made on axes is constant. Show that the line passes through a fixed point.
In what direction should a line be drawn through the point (1, 2) so that its point of intersection with the line x + y = 4 is at a distance √6/3 from the given point.
A variable line passes through a fixed point P. The algebraic sum of the perpendiculars drawn from the points (2, 0), (0, 2) and (1, 1) on the line is zero. Find the coordinates of the point P.
\[\begin{array}{*{35}{l}} \Rightarrow ~2a\text{ }-\text{ }1\text{ }+\text{ }2b\text{ }-\text{ }1\text{ }+\text{ }a\text{ }+\text{ }b\text{ }-\text{ }1\text{ }=\text{ }0 \\ \Rightarrow ~3a\text{...
If the equation of the base of an equilateral triangle is x + y = 2 and the vertex is (2, – 1), then find the length of the side of the triangle.
Find the equation of one of the sides of an isosceles right angled triangle whose hypotenuse is given by 3x + 4y = 4 and the opposite vertex of the hypotenuse is (2, 2).
Find the equation of a straight line on which length of perpendicular from the origin is four units and the line makes an angle of 120° with the positive direction of x-axis.
intercept of a line between the coordinate axes is divided by the point (–5, 4) in the ratio 1:2, then find the equation of the line.
If 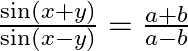 , then show that
, then show that 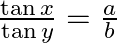 [Hint: Use componendo and Dividendo]
[Hint: Use componendo and Dividendo]
Solution: As per the question, $\frac{\sin (x+y)}{\sin (x-y)}=\frac{a+b}{a-b}$ $\text { Since, } \sin (A+B)=\sin A \cos B+\cos A \sin B$ $\therefore \frac{\sin (x+y)}{\sin (x-y)}=\frac{a+b}{a-b}$...
For what values of a and b the intercepts cut off on the coordinate axes by the line ax + by + 8 = 0 are equal in length but opposite in signs to those cut off by the line 2x – 3y + 6 = 0 on the axes.
Show that the first few frequencies of light that are emitted when electrons fall to the 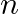 th level from levels higher than
th level from levels higher than 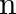 are approximate harmonics (i.e. in the ratio
are approximate harmonics (i.e. in the ratio 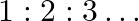 ) when
) when 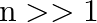 .
.
The difference between the two atoms is used to indicate the frequency of any line in the hydrogen spectrum series. $\mathrm{f_{cm}}=\mathrm{cRZ}^{2}\left[1 /(\mathrm{n}+\mathrm{p})^{2}-1 /...
Find the equation of lines passing through (1, 2) and making angle 30° with y-axis.
SOLUTION: Given that line passing through (1, 2) making an angle 30° with y – axis. Angle made by the line with x – axis is (90° – 30°) = 60° ∴ Slope of the line, m = tan 60° = √3 So, the equation...
If 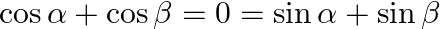 , then prove that
, then prove that 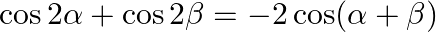 .
. ![Rendered by QuickLaTeX.com \left[\text { Hint: } \cos \alpha+\cos \beta)^{2}-(\sin \alpha+\sin \beta)^{2}=0\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-23550638ec8563c80886fdab1ac1f548_l3.png)
Solution: As per the question, $\cos \alpha+\cos \beta=0=\sin \alpha+\sin \beta \ldots \dots(i)$ As, $\text {Left Hand Side} =\cos 2 \alpha+\cos 2 \beta$ It is known that, $\cos 2 x=\cos ^{2} x-\sin...
Assume that there is no repulsive force between the electrons in an atom but the force between positive and negative charges is given by Coulomb’s law as usual. Under such circumstances, calculate the ground state energy of a He-atom.
For He nucleus we have, $Z=2$ and in ground state, $n=1$ As a result, $E n=-13.6 Z^{2} / n^{2} e V=-54.4 \mathrm{eV}$
Show that the tangent of an angle between lines x/a+y/b=1 and x/a-y/b=1 is 2ab/a^2-b^2
Consider two different hydrogen atoms. The electron in each atom is in an excited state. Is it possible for the electrons to have different energies but the same orbital angular momentum according to the Bohr model?
Because the value of n in two hydrogen atoms differs, their angular momentum differs as well. The angular momentum, according to Bohr's model, is given as L = nh/2π
Find the points on the line x + y = 4 which lie at a unit distance from the line 4x + 3y = 10.
Putting the value of y1 = 4 – x1 in equation 3, we get \[\begin{array}{*{35}{l}} 4{{x}_{1}}~+\text{ }3\left( 4\text{ }-\text{ }{{x}_{1}} \right)\text{ }=\text{ }5 \\ \Rightarrow ~4{{x}_{1}}~+\text{...
Find the equation of the lines which passes through the point (3, 4) and cuts off intercepts from the coordinate axes such that their sum is 14.
Find the angle between: y=2-√3(x+5) and y=2+√3(x-7)
Find the equation of the line passing through the point (5, 2) and perpendicular to the line joining the points (2, 3) and (3, – 1).
The points are A (5, 2), B (2, 3) and C (3, -1)
Find the equation of the straight line which passes through the point (1, – 2) and cuts off equal intercepts from axes.
If tan (A + B) = p, tan (A – B) = q, then show that tan 2A = (p + q) / (1 – pq). [Hint: Use 2A = (A + B) + (A – B)]
Solution: It is known that, $\tan 2 A=\tan (A+B+A-B)$ Also, $\tan (x+y)=\frac{\tan x+\tan y}{1-\tan x \tan y}$ $\therefore \tan 2 A=\frac{\tan (A+B)+\tan (A-B)}{1-\tan (A+B) \tan (A-B)}$ Now...
Consider aiming a beam of free electrons towards free protons. When they scatter, an electron and a proton cannot combine to produce an H-atom,
(a) because of energy conservation
(b) without simultaneously releasing energy in the form of radiation
(c) because of momentum conservation
(d) because of angular momentum conservation
The correct options are: (a) because of energy conservation (b) without simultaneously releasing energy in the form of radiation
An ionised H-molecule consists of an electron and two protons. The protons are separated by a small distance of the order of angstrom. In the ground state,
(a) the electron would not move in circular orbits
(b) the energy would be (2) 4 times that of an 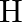 -atom
-atom
(c) the electrons, the orbit would go around the protons
(d) the molecule will soon decay in a proton and an 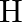 -atom
-atom
The correct options are: (a) the electron would not move in circular orbits (c) the electrons, the orbit would go around the protons
Two H atoms in the ground state collide inelastically. The maximum amount by which their combined kinetic energy is reduced is
(a) 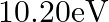
(b) 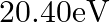
(c) 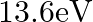
(d) 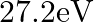
The correct option is: (a) $10.20 \mathrm{eV}$
If 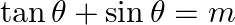 and
and 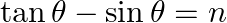 , then prove that
, then prove that 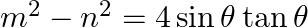 [Hint:
[Hint: 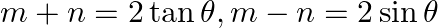 , then use
, then use ![Rendered by QuickLaTeX.com \left.m^{2}-n^{2}=(m+n)(m-n)\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-605feb2e4429368843fd21e15ecb1047_l3.png)
Solution: As per the question, $\tan \theta+\sin \theta=m \ldots \dots$...(i) $\tan \theta-\sin \theta=n \ldots \dots$ (ii) Now add eq.(i) and eq.(ii), $2 \tan \theta=m+n \ldots \dots$ (iii) Now...
The simple Bohr model cannot be directly applied to calculate the energy levels of an atom with many electrons. This is because
(a) of the electrons not being subject to a central force
(b) of the electrons colliding with each other
(c) of screening effects
(d) the force between the nucleus and an electron will no longer be given by Coulomb’s law
The correct option is: (a) of the electrons not being subject to a central force
Prove that 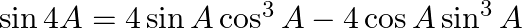
Solution: $\sin 4 A=\sin (2 A+2 A)$ It is known that, $\sin (A+B)=\sin A \cos B+\cos A \sin B$ As a result, $\sin 4 A=\sin 2 A \cos 2 A+\cos 2 A \sin 2 A$ $\Rightarrow \sin 4 A=2 \sin 2 A \cos 2 A$...
The binding energy of an H-atom, considering an electron moving around a fixed nucleus (proton), is 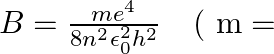 electron mass
electron mass 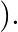 If one decides to work in a frame of reference where the electron is at rest, the proton would be moving around it. By similar arguments, the binding energy would be
If one decides to work in a frame of reference where the electron is at rest, the proton would be moving around it. By similar arguments, the binding energy would be 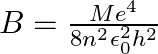 (M = proton mass) This last expression is not correct because
(M = proton mass) This last expression is not correct because
(a) n would not be integral
(b) Bohr-quantisation applies only to electron
(c) the frame in which the electron is at rest is not inertial
(d) the motion of the proton would not be in circular orbits, even approximately
The correct option is: (c) the frame in which the electron is at rest is not inertial
Find the value of 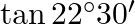 . [Hint: Let
. [Hint: Let 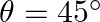 , use
, use ![Rendered by QuickLaTeX.com \left.\tan \frac{\theta}{2}=\frac{\sin \frac{\theta}{2}}{\cos \frac{\theta}{2}}=\frac{2 \sin \frac{\theta}{2} \cos \frac{\theta}{2}}{2 \cos ^{2} \frac{\theta}{2}}=\frac{\sin \theta}{1+\cos \theta}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-00a87a317270f0f1bbc1f2d0792947a5_l3.png)
Solution: Let's say, $\theta=45^{\circ}$ To find: $\tan 22^{\circ} 30^{\prime}=\tan (\theta / 2)$ It is known that, $\sin \theta=\cos \theta=1 / \sqrt{2}\left(\right.$ for...
Nuclei with magic no. of proton 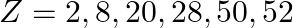 and magic no. of neutrons
and magic no. of neutrons 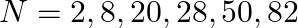 and
and 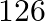 are found to be very stable
are found to be very stable
(i) Verify this by calculating the proton separation energy Sp for 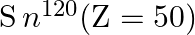 and
and 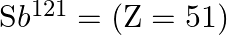 . The proton separation energy for a nuclide is the minimum energy required to separate the least tightly bound proton from a nucleus of that nuclide. It is given by
. The proton separation energy for a nuclide is the minimum energy required to separate the least tightly bound proton from a nucleus of that nuclide. It is given by 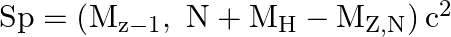 . Given
. Given 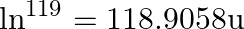 ,
, 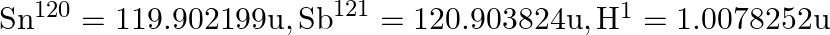
(ii) What does the existence of magic number indicate?
i) The proton separation energy is given by, $\mathrm{SpSn}=(\mathrm{M} 119.70+\mathrm{Mh}-\mathrm{M} 120.70) \mathrm{c}^{2}=0.0114362 \mathrm{c}^{2}$ Similarly we have, $\mathrm{SpSp}=(\mathrm{M}...
If a cos θ + b sin θ = m and a sin θ – b cos θ = n, then show that 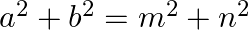 .
.
Solution: As per the question, $a \cos \theta+b \sin \theta=m \ldots \dots(i)$ $a \sin \theta-b \cos \theta=n \ldots \dots(ii)$ Now square and add eq.1 and eq.2, we have, $(a \cos \theta+b \sin...
Prove that cos θ cos θ/2 – cos 3θ cos 9θ/2 = sin7θ sin4θ [Hint: Express L.H.S. = ½ [2cos θcos θ/2 – 2cos 3θ cos 9θ / 2]
Solution: By applying transformation formula, we have, $2 \cos A \cos B=\cos (A+B)+\cos (A-B)$ $-2 \sin A \sin B=\cos (A+B)-\cos (A-B)$ Now multiply and divide the expression by 2 . $\therefore...
If 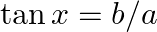 then find the value of
then find the value of 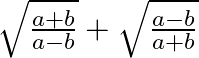
Solution: As per the question, $\tan x=b / a$ Let's say, $y=\sqrt{\frac{a+b}{a-b}}+\sqrt{\frac{a-b}{a+b}}$ $\therefore y=\sqrt{\frac{a\left(1+\frac{b}{a}\right)}{a\left(1-...
If 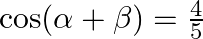 and
and 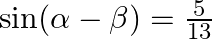 , where a lie between 0 and
, where a lie between 0 and 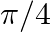 , find value of
, find value of 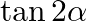 [Hint: Express tan
[Hint: Express tan 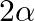 as tan
as tan ![Rendered by QuickLaTeX.com (\alpha+\beta+\alpha-\beta]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0c8609776d53ba617c166550ac3fb3d9_l3.png)
Solution: As per the question, $\cos (\alpha+\beta)=4 / 5 \ldots \text {...(i) }$ It is known that, $\sin x=\sqrt{\left(1-\cos ^{2} x\right)}$ So, $\begin{array}{l} \sin...
If m sin θ = n sin (θ + 2α), then prove that tan (θ + α) cot α = (m + n)/(m – n) [Hints: Express sin(θ + 2α) / sinθ = m/n and apply componendo and dividend]
Solution: As per the question, $m \sin \theta=n \sin (\theta+2 a)$ We need to prove: $\tan (\theta+\alpha) \cot \alpha=(m+n) /(m-n)$ Proof: $\begin{array}{l} m \sin \theta=n \sin (\theta+2 a) \\...
If [2sinα / (1+cosα+sinα)] = y, then prove that [(1– cosα+sinα) / (1+sinα)] is also equal to y. Hint: Express ![Rendered by QuickLaTeX.com \left.\frac{1-\cos \alpha+\sin \alpha}{1+\sin \alpha}=\frac{1-\cos \alpha+\sin \alpha}{1+\sin \alpha} \cdot \frac{1+\cos \alpha+\sin \alpha}{1+\cos \alpha+\sin \alpha}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-2dd16b460a40b6c1bae1bc04a74f878a_l3.png)
Solution: As per the question, $y=2 \sin \alpha /(1+\cos \alpha+\sin \alpha)$ Now multiply the numerator and denominator by $(1-\cos \alpha+\sin \alpha)$, We have, $\Rightarrow y=\frac{2 \sin...
Before the neutrino hypothesis, the beta decay process was thought to be the transition 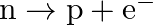 . If this was true, show that if the neutron was at rest, the proton and electron would emerge with fixed energies and calculate them. Experimentally, the electron energy was found to have a large range.
. If this was true, show that if the neutron was at rest, the proton and electron would emerge with fixed energies and calculate them. Experimentally, the electron energy was found to have a large range.
It is given that neutron was at rest before $\beta$ decay from neutron. So, energy of neutron $=$ $\mathrm{E}_{\mathrm{n}}=\mathrm{m}_{\mathrm{n}} \mathrm{c}^{2}$. Momentum of neutron...
Prove that 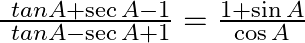
Solution: As per the question, $\text { Left Hand Side }=\frac{\ tan A+\sec A-1}{\ tan A-\sec A+1}$ $=\frac{\frac{\sin A}{\cos A}+\frac{1}{\cos A}-1}{} {\frac{\sin A}{\cos A}-\frac{1}{\ cos A}+1}$...
The deuteron is bound by nuclear forces just as 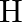 -atom is made up of
-atom is made up of 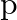 and e bound by electrostatic forces. If we consider the force between neutron and proton in deuteron as given in the form of a Coulomb potential but with an effective charge
and e bound by electrostatic forces. If we consider the force between neutron and proton in deuteron as given in the form of a Coulomb potential but with an effective charge 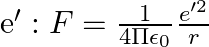 estimate the value of
estimate the value of 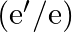 given that the binding energy of a deuteron is
given that the binding energy of a deuteron is 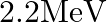 .
.
The binding energy of $\mathrm{H}$ atom is given as $\mathrm{E}=13.6 \mathrm{eV}$ The reduced $\mathrm{m}^{\prime}$ is given as $918 \mathrm{~m}$ The mass of a neutron or a proton is given as...
Deuteron is a bound state of a neutron and a proton with a binding energy B = 2.2 MeV. A γ -ray of energy E is aimed at a deuteron nucleus to try to break it into a (neutron + proton) such that the n and p move in the direction of the incident γ-ray. If E = B, show that this cannot happen. Hence calculate how much bigger than B must E be for such a process to happen.
The binding energy of a deuteron is given as $B = 2.2 MeV$ The kinetic energies of neutron and proton be $K_n$ and $K_p$ $p_n$ and $p_p$ are the momentum of neutron and proton $E - B = K_n + K_p$ B...
Sometimes a radioactive nucleus decays into a nucleus which itself is radioactive. An example is
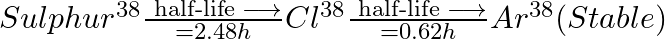
Assume that we start with 1000 38S nuclei at time  . The number of
. The number of 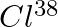 is of count zero at
is of count zero at  and will again be zero at
and will again be zero at 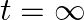 . At what value of
. At what value of 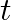 , would the number of counts be a maximum?
, would the number of counts be a maximum?
Let the disintegration constants for $S^{38}$ and $Cl^{38}$ be $\lambda_ 1$ and $\lambda_ 2$ respectively. $\mathrm dN_1 / \mathrm{dt}=-\lambda N_{1}$ $\mathrm dN_2 / \mathrm{dt}=$ rate of decay of...
Are the nucleons fundamental particles, or do they consist of still smaller parts? One way to find out is to probe a nucleon just as Rutherford probed an atom. What should be the kinetic energy of an electron for it to be able to probe a nucleon? Assume the diameter of a nucleon to be approximately 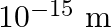 .
.
$\lambda=\mathrm{h} / \mathrm{p}$ and, kinetic energy $=$ potential energy $\mathrm{E}=\mathrm{hc} / \lambda$ Kinetic energy of an electron can be calculated as, $\mathrm{KE}=\mathrm{PE}=\mathrm{hc}...
A piece of wood from the ruins of an ancient building was found to have a 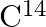 activity of 12 disintegrations per minute per gram of its carbon content. The
activity of 12 disintegrations per minute per gram of its carbon content. The 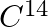 activity of the living wood is 16 disintegrations per minute per gram. How long ago did the tree, from which the wooden sample came, die? Given the half-life of
activity of the living wood is 16 disintegrations per minute per gram. How long ago did the tree, from which the wooden sample came, die? Given the half-life of 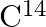 is 5760 years.
is 5760 years.
$\mathrm{C}^{14}$ activity of a piece of wood from the ruins is given as $\mathrm{R}=12 \mathrm{dis} / \mathrm{min}$ per gram $\mathrm{C}^{14}$ activity of a living wood is given as...
Consider a radioactive nucleus A which decays to a stable nucleus 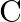 through the following sequence:
through the following sequence: 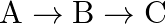 Here
Here 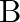 is an intermediate nuclei which is also radioactive. Considering that there are
is an intermediate nuclei which is also radioactive. Considering that there are 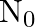 atoms of A initially, plot the graph showing the variation of number of atoms of
atoms of A initially, plot the graph showing the variation of number of atoms of 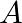 and
and 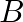 versus time.
versus time.
At $\mathrm{t}=0,$ $\mathrm{~N}_{\mathrm{A}}=\mathrm{N}_{0}$ As time passes, $N_A$ decreases exponentially, while the number of atoms in B increases, reaches its maximum, and then decays to...
In pair annihilation, an electron and a positron destroy each other to produce gamma radiation. How is the momentum conserved?
An electron and a positron kill one other to produce gamma radiation in pair annihilation. Also, their momentum is conserved because they move in opposing directions.
Which one of the following cannot emit radiation and why? Excited nucleus, excited electron.
Because the energy of the electronic energy level is in the eV range rather than the MeV range, an excited electron cannot release radiation.
Which sample, A or B shown in the figure has shorter mean-life?
Solution: At t=0, $(d N / d t) A=(d N / d t) B$ $d N / d t=-\lambda N$ $\left(N_{0}\right) A=\left(N_{0}\right) B$ $\lambda_ A N_ A=\lambda_ B N_ B$ $N_ a>N_ B$ $\lambda_ B>\lambda_ A$
