Because energy is required to overcome the attractions between the haloalkane molecules as well as to break the hydrogen bonds between water molecules, haloalkanes are only weakly soluble in water.
Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
Electrophilic substitution can be used to make aryl bromides and chlorides from arenes. In the absence of light, this reaction is carried out by treating the arene with chlorine or bromine in the...
Haloarenes are less reactive than haloalkanes and haloalkenes. Explain.
This is due to the aryl ring's resonance stabilisation. There will be a conjugation of chlorine electrons with the electrons in the ring in the case of C6H5-Cl. The partial double bond nature of the...
Why iodoform has appreciable antiseptic property?
Iodoform has a significant antibacterial activity due to the release of free iodine.
Which of the compounds will react faster in SN1 reaction with the –OH ion? CH3— CH2— Cl or C6H5— CH2— Cl
In an SN1 reaction with the OH- ion, C6H5— CH2— Cl will react quicker. This is owing to the carbocation's stability in the compound. The C6H5 group is already stable owing to resonance, and the CH2...
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts. But why does the preparation of aryl iodides requires the presence of an oxidising agent?
Arenes' iodination can be reversed due to the production of HI. An oxidising agent, such as HNO3 or HIO4, oxidises HI to speed up the process and stabilise the result.
Alkyl fluorides are synthesised by heating an alkyl chloride/bromide in presence of ____________ or ____________. (i) Ca F2 (ii) CoF2 (iii) Hg2F2 (iv) NaF
Option (ii) and (iii) are the answers. Heating an alkyl chloride or bromide in the presence of a metallic fluoride such as AgF, FIg2F2, CoF2, or SbF3 results in the production of alkyl fluorides....
Alkyl halides are prepared from alcohols by treating with (i) HCl + ZnCl2 (ii) Red P + Br2 (iii) H2SO4+ KI (iv) All the above
Option (i) and (ii) are the answers (i) $R-\mathrm{OH} \stackrel{\mathrm{HCl}+\mathrm{ZnCl}}{\longrightarrow} R-\mathrm{Cl}_{2}+\mathrm{H}_{2} \mathrm{O}$ (ii) $R-\mathrm{OH}...
Which of the following are secondary bromides? (i) (CH3)2 CHBr (ii) (CH3)3C CH2Br (iii) CH3CH(Br)CH2CH3 (iv) (CH3)2CBrCH2CH3
Option (i) and (iii) are the answers. Secondary bromides are those in which the -carbone (bromine-bound carbon) is further attached to two alkyl groups. The -carbon in compounds (i) and (iii) is...
Ethylene chloride and ethylidene chloride are isomers. Identify the correct statements. (i) Both the compounds form the same product on treatment with alcoholic KOH. (ii) Both the compounds form the same product on treatment with aq.NaOH. (iii) Both the compounds form the same product on reduction. (iv) Both the compounds are optically active.
Option (i) and (iii) are the answers. They give ethyne on treatment with alcoholic $\mathrm{KOH}$. $ \mathrm{CH}_{3} \mathrm{CHCl}_{2} \underset{\mathrm{KOH}}{\stackrel{\text { alc....
Haloalkanes contain halogen atom (s) attached to the sp3 hybridised carbon atom of an alkyl group. Identify haloalkane from the following compounds. (i) 2-Bromopentane (ii) Vinyl chloride (chloroethene) (iii) 2-chloroacetophenone (iv) Trichloromethane
Option (i) and (iv) are the answers. Halogen atoms are linked to the sp3 hybridised carbon atom of the alkyl group in each of these molecules.
Which of the following statements are correct about the mechanism of this reaction? (i) A carbocation will be formed as an intermediate in the reaction. (ii) OH–will attach the substrate (b) from one side and Cl- will leave it simultaneously from the other side. (iii) An unstable intermediate will be formed in which OH– and Cl– will be attached by weak bonds. (iv) The reaction proceeds through an SN1 mechanism.
Option (i) and (iv) are the answers. Because it is a tertiary halide, it undergoes the SN1 process, resulting in the formation of a carbocation as an intermediate.
Which of the following statements are correct about the reaction intermediate? (i) Intermediate (c) is unstable because in this carbon is attached to 5 atoms. (ii) Intermediate (c) is unstable because carbon atom is sp2 hybridised. (iii) Intermediate (c) is stable because carbon atom is sp2 hybridised. (iv) Intermediate (c) is less stable than the reactant (b).
Option (i) and (iv) are the answers. Intermediate (iii) is unstable in the above reaction because the carbon atom is linked to 5 atoms and is less stable than reactant (ii).
Which of the following statements are correct about this reaction? (i) The given reaction follows the SN2 mechanism. (ii) (b) and (d) have the opposite configuration. (iii) (b) and (d) have the same configuration. (iv) The given reaction follows the SN1 mechanism.
Option (i) and (ii) are the answers. Alkyl halide is the main reactant in the given reaction. A transient condition is found here, in which one bond is broken and another is created synchronously,...
Which of the statements are correct about the above reaction? (i) (a) and (e) both are nucleophiles. (ii) In (c) carbon atom is sp3 hybridised. (iii) In (c) carbon atom is sp2 hybridised. (iv) (a) and (e) both are electrophiles.
Option (i) and (iii) are the answers. Nucleophiles are HO and CF. The C atom is sp2 hybridised in (iii) due to the simultaneous creation of the C – OH bond and the breakdown of the C – Cl link. As a...
Which is the correct increasing order of boiling points of the following compounds? 1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene (i) Bromobenzene < 1-Bromobutane < 1-Bromopropane < 1-Bromoethane (ii) Bromobenzene < 1-Bromoethane < 1-Bromopropane < 1-Bromobutane (iii) 1-Bromopropane < 1-Bromobutane < 1-Bromoethane < Bromobenzene (iv) 1-Bromoethane < 1-Bromopropane < 1-Bromobutane < Bromobenzene
Option (iv) is the answer Reason: As the molecular mass of the alkyl halide grows, the boiling point rises.
Which is the correct increasing order of boiling points of the following compounds? 1-Iodobutane, 1-Bromobutane, 1-Chlorobutane, Butane (i) Butane < 1-Chlorobutane < 1-Bromobutane < 1-Iodobutane (ii) 1-Iodobutane < 1-Bromobutane < 1-Chlorobutane < Butane (iii) Butane < 1-Iodobutane < 1-Bromobutane < 1-Chlorobutane (iv) Butane < 1-Chlorobutane < 1-Iodobutane < 1-BromobutaneSolution:
Option (i) is the answer. Explanation: The larger the surface area, the stronger the intermolecular forces of attraction and, as a result, the higher the boiling point. For comparable types of alkyl...
arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.(i) (c) < (b) < (a) (ii) (b) < (c) < (a) (iii) (a) < (c) < (b) (iv) (a) < (b) < (c)
Option (iv) is the answer. Electron withdrawing groups boost aryl halide reactivity; the higher the number of electron withdrawing groups, the higher the rate of nucleophilic substitution.
arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.(i) (a) < (b) < (c) (ii) (a) < (c) < (b) (iii) (c) < (b) < (a) (iv) (b) < (c) < (a)
Option (iv) is the answer. The presence of an electron-releasing group in the ortho or para locations slows down nucleophilic substitution.
arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.(i) (a) < (b) < (c) (ii) (c) < (b) < (a) (iii) (a) < (c) < (b) (iv) (c) < (a) < (b)
Option (iii) is the answer. Nucleophilic substitution is facilitated by the presence of an electron withdrawing group (-NO2) in the ortho and para positions. At meta position, the presence of an...
Which of the carbon atoms present in the molecule given below are asymmetric?(i) a, b, c, d (ii) b, c (iii) a, d (iv) a b, c
Option (ii) is the answer. The chiral carbon, which is $sp_3$ hybridised carbon attached to four distinct substituents, is known as asymmetric carbon. Because (b) and (c) are $sp_3$ hybridised, they...
Chloromethane on treatment with an excess of ammonia yields mainly
Option (iii) is the answer. After reacting with $NH_3$ and chloromethane, methanamine is formed.
The reaction of toluene with chlorine in the presence of iron and the absence of light yields ____________.
Option (d) is the answer. In the presence of iron and in the absence of light, toluene reacts with chlorine to produce a mixture of 1-chloro-2-methylbenzene and 1-chloro-4-methylbenzene. The methyl...
Which of the following alkyl halides will undergo SN1 reaction most readily? (i) (CH3)3C—F (ii) (CH3)3C—Cl (iii) (CH3)3C—Br (iv) (CH3)3C—I
Option (iv) is the answer. SN1 reactions occur mostly in polar protic solvents such as H2O and follow first-order kinetics. This indicates that the reaction rate is solely determined by one...
A primary alkyl halide would prefer to undergo _____________. (i) SN1 reaction (ii) SN2 reaction (iii) α–Elimination (iv) Racemisation
Option (ii) is the answer. Primary alkyl halides undergo SN2 mechanisms because 1∘ substrates have little steric hindrance to nucleophilic attack and 1∘ carbocations are relatively unstable....
What is ‘A’ in the following reaction?
The solution is option (iii). The addition of HCl across the double bond follows the Markovnikov rule in the aforementioned reaction. The negative portion of the addition molecule is linked to the...
Ethylidene chloride is a/an ______________. (i) vic-dihalide (ii) gem-dihalide (iii) allylic halide (iv) vinylic halide
Option (ii) is the answer. A gem-dihalide is ethylidene chloride. A compound with two halogen atoms on the same carbon atom is known as Gem Dihalide.
Chlorobenzene is formed by the reaction of chlorine with benzene in the presence of 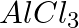 . Which of the following species attacks the benzene ring in this reaction? (i) Cl– (ii) Cl+ (iii)
. Which of the following species attacks the benzene ring in this reaction? (i) Cl– (ii) Cl+ (iii)  (iv) [AlCl4]–
(iv) [AlCl4]–
Option (ii) is the answer. Chlorobenzene is formed by the reaction of chlorine with benzene in the presence of AlCl3. Cl+ attacks the benzene ring in this reaction.
The position of –Br in the compound in CH3CH==CHC(Br)(CH3)2 can be classified as ____________. (i) Allyl (ii) Aryl (iii) Vinyl (iv) Secondary
Optuion (i) is the answer. A) Allyl : $\mathrm{CH}_{3} \mathrm{CH}=\mathrm{CHC}(\mathrm{Br})\left(\mathrm{CH}_{3}\right)_{2}$ B) Aryl: $\mathrm{p}-\mathrm{ClC}_{6} \mathrm{H}_{4} \mathrm{CH}_{2}...
Which of the following is an example of vic-dihalide? (i) Dichloromethane (ii) 1,2-dichloroethane (iii) Ethylidene chloride (iv) Allyl chloride
Option (ii) is the answer. Vicinal dihalides are formed when a halogen reacts with an alkene to form compounds with halogen on neighbouring carbons. In terms of yearly output, 1, 2- dichloroethane...
Which of the following structures is enantiomeric with the molecule (A) given below :
Option (i) is the answer. Enantiomers are a pair of molecules that are mirror copies of one another.
In which of the following molecules carbon atom marked with an asterisk (*) is asymmetric?(i) (a), (b), (c), (d) (ii) (a), (b), (c) (iii) (b), (c), (d) (iv) (a), (c), (d)
Option (ii) is the answer. Asymmetric carbon atoms are those that have four distinct groups or atoms connected to them. The compounds a,b,c satisfy this criterion, thus B is the right answer.
Arrange the following compounds in increasing order of their boiling points.(i) (b) < (a) < (c) (ii) (a) < (b) < (c) (iii) (c) < (a) < (b) (iv) (c) < (b) < (a)
Option (iii) is the answer. The boiling point of an alkyl halide falls as the branching rises. As a result, tert butyl bromide has the lowest boiling point while n butyl bromide has the greatest...
Arrange the following compounds in the increasing order of their densities.(i) (a) < (b) < (c) < (d) (ii) (a) < (c) < (d) < (b) (iii) (d) < (c) < (b) < (a) (iv) (b) < (d) < (c) < (a)
Option (i) is the answer. Reason:The density of a substance is exactly proportional to its molar mass at constant volume.
Which reagent will you use for the following reaction? CH3CH2CH2CH3 → CH3CH2CH2CH2Cl + CH3CH2CHClCH3 (i) Cl2/UV light (ii) NaCl + H2SO4 (iii) Cl2 gas in dark (iv) Cl2 gas in the presence of iron in dark
Option (i) is the answer. Mono-chlorinated isomeric products of Cl 2/UV light free radical substitution
Which of the following is the halogen exchange reaction?
Option (i) is the answer. Metal–halogen exchange is a basic reaction in organometallic chemistry that transforms an organic halide into an organometallic product. Electropositive metals (Li, Na, Mg)...
Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is (i) Electrophilic elimination reaction (ii) Electrophilic substitution reaction (iii) Free radical addition reaction (iv) Nucleophilic substitution reaction
Option (ii) is the answer. In the presence of Iron(III) chloride, toluene interacts with halogen to produce ortho and para halo compounds in an electrophilic substitution process. The Cl atom...
Identify the compound Y in the following reaction.
Option (i) is the answer.
Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated HCl at room temperature?
Option (iv) is the answer. If any alkyl halide forms a more stable carbocation at room temperature, the result is a more stable and efficient product. The formation of a $3^0$ carbocation as an...
Write the structure of the major organic product in each of the following reactions:
Solution:
Write the structure of the major organic product in each of the following reactions:
Solution: (i) (ii)
What happens when (i) methyl bromide is treated with sodium in the presence of dry ether, (Ii) methylchloride is treated with KCN.
(i) Ethane is generated when methyl bromide is reacted with sodium in the presence of dry ether. The Wurtz reaction is the name for this response. (ii)
What happens when (i) chlorobenzene is subjected to hydrolysis, (ii) ethyl chloride is treated with aqueous KOH
(i) Under normal circumstances, chlorobenzene does not hydrolyze. When heated in an aqueous sodium hydroxide solution at a temperature of 623 K and a pressure of 300 atm, it hydrolyzes to produce...
What happens when (i) n-butyl chloride is treated with alcoholic KOH, (ii) bromobenzene is treated with Mg in the presence of dry ether.
(i) The production of but-1-ene occurs when n-butyl chloride is treated with alcoholic KOH. A dehydrohalogenation process is what this is. (ii) Phenylmagnesium bromide is generated when bromobenzene...
Primary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b). Compound (b) is reacted with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it gives compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
The formula C4H9Br is used to make two main alkyl halides. They're n-butyl bromide and isobutyl bromide, respectively. Compound (a) is therefore either nbutyl bromide or isobutyl bromide. Compound...
The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
KOH almost entirely ionizes in an aqueous solution, yielding OH ions. Because the OH ion is a powerful nucleophile, it causes the alkyl chloride to undergo a substitution reaction, resulting in the...
p-Dichlorobenzene has higher m.p. and lower solubility than those of o- and m-isomers. Discuss.
The symmetrical nature of p-Dichlorobenzene is superior to that of the o- and m-isomers. As a result, it matches the crystal lattice better than the o- and m-isomers. As a result, breaking the...
Out of C6H5CH2Cl and C6H5CHClC6H5, which is more easily hydrolyzed by aqueous KOH?
The production of carbocation occurs during aqueous KOH hydrolysis. If the carbocation is stable, aqueous KOH can easily hydrolyze the compound. Now, C6H5 CH2Cl creates 1o– carbohydrate, whereas...
Arrange the compounds of each set in order of reactivity towards SN2 displacement: (i) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane (ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 3-Bromo-2- methylbutane (iii) 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane, 1Bromo-3- methylbutane.
(i) The nucleophile approaches the carbon atom to which the leaving group is linked in an SN2 reaction. The reactivity for SN2 displacement reduces when the nucleophile is sterically inhibited. The...
What will be the mechanism for the following reaction?
Solution: The given reaction is CN- functions as a nucleophile, attacking the carbon atom that the Br is bonded to. The CN– ion is a nucleophile that can attack at both the C and N sites. In this...
Write the structure of the major organic product in each of the following reactions:
Solution:
Write the structure of the major organic product in each of the following reactions:
Solution:
Give the uses of freon 12, DDT, carbon tetrachloride and iodoform.
Freon -12 has a variety of applications. Freon-12 (dichlorodifluoromethane, CF2Cl2) is also known as CFC. It's found in deodorants, hair sprays, and other aerosol spray propellants. It's also...
Explain why (i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (ii) alkyl halides, though polar, are immiscible with water? (iii) Grignard reagents should be prepared under anhydrous conditions?
(i) The Cl- atom in chlorobenzene is coupled to an sp2 hybridized carbon atom, whereas it is linked to an sp3 hybridized carbon atom in cyclohexyl chloride. The sp2 hybridized carbon atom is now...
How will you bring about the following conversions? (i) 1-Chlorobutane to n-octane (ii) Benzene to biphenyl.
(i) (ii)
How will you bring about the following conversions? (i) Bromomethane to propanone (ii) But-1-ene to but-2-ene
(i) (ii)
How will you bring about the following conversions? (i) Propene to propyne (ii) Ethanol to ethyl fluoride
(i) (ii)
How will you bring about the following conversions? (i) Propene to 1-nitropropane (ii) Toluene to benzyl alcohol
(i) (ii)
How will you bring about the following conversions? (i) Ethanol to but-1-yne (ii) Ethane to bromoethene
(i) (ii)
Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene: (i) 1-Bromo-1-methylcyclohexane (ii) 2-Chloro-2-methyl butane (iii) 2,2,3-Trimethyl-3-bromopentane.
(i) 1−bromo−1−methylcyclohexane In the given molecule, all β-hydrogens are equivalent. As a result, only one alkene is produced when the given molecule is dehydrogenated. (ii) Different sets of...
Which compound in each of the following pairs will react faster in SN2 reaction with –OH? (i) CH3Br or CH3I (ii) (CH3)3CCl or CH3Cl
(i) The order in which the halides react to an alkyl group is constant in the SN2 process. This is because as the size of the halide ion rises, it becomes a better leaving group. R-F << R-Cl...
What are ambident nucleophiles? Explain with an example.
The term ambident nucleophile refers to a nucleophile with two nucleophilic sites. These nucleophilic sites are targets for them to attack. For example, the nitrite ion. When the nitrite ion can...
Write the equations for the preparation of 1−iodobutane from: (i) 1-butanol (ii) 1-chlorobutane (iii) but-1-ene
(i) (ii) (iii)
Write the isomers of the compound having formula C4H9Br.
(i) 1−Bromobutane (ii) 2−Bromobutane (iii) 1−Bromo−2−methylpropane (iv) 2−Bromo−2−methylpropane
A hydrocarbon C5H10does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
C5H10 is a hydrocarbon with the chemical formula CnH2n, which belongs to the CnH2n group of hydrocarbons. As a result, it could be an alkene or a cycloalkane. Hydrocarbon cannot be an alkene because...
Give the IUPAC names of the following compounds:
(i) CH3 C (p – Cl C6 H4 )2 CH(Br) CH3 (ii) (CH3 )3 C CH = C Cl C6 H4 I – p (i) 2−Bromo−3, 3−bis(4 − chlorophenyl) butane (ii) 1−chloro−1−(4−iodophenyl)−3,...
Give the IUPAC names of the following compounds:
(i) Cl CH2 C ≡ C CH2 Br (iv) ( CCl 3) 3 CCl (i) 1 – Bromo – 4 – chlorobut – 2 – yne (ii) 2−(Trichloromethyl)−1,1,1,2,3,3,3−heptachloropropane
Give the IUPAC names of the following compounds:
(i) CH3 CH(Cl) CH(Br) CH3 (ii) CH F2 CBr ClF Solution: (i) 2−Bromo−3−chlorobutane (ii) 1−Bromo−1−chloro−1, 2, 2−trifluoroethane
Which one of the following has the highest dipole moment?
(i) CH2Cl2 (ii) CHCl3 (iii) CCl4 Solution: In CHCl3, the resultant of dipole moments of two C – Cl bonds is countered by the resultant of dipole moments of one CH bond and one C – Cl bond, as seen...
Write the structures of the following organic halogen compounds.
(i) 1 – Bromo – 4 – sec – butyl – 2 – methylbenzene (ii) 1 ,4 – Dibromobut – 2 – ene Solution: (i) (ii)
Write the structures of the following organic halogen compounds.
(i) 2 – Bromobutane (ii) 4 – tert – Butyl -3 –iodoheptane Solution: (i) (ii)
Write the structures of the following organic halogen compounds.
(i) 1 -Chloro-4-ethylcyclohexane (ii) 2 – (2 -Chlorophenyl) -1 –iodooctane Solution: (i) (ii)
Write the structures of the following organic halogen compounds.
(i) 2 -Chloro-3 -methylpentane (ii) p -Bromochlorobenzene Solution: (i) (ii)
Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl ( primary, secondary, tertiary ), vinyl, or aryl halides.
(i) m-ClCH2C6H4CH2C(CH3)3 (ii) o-Br-C6H4CH(CH3)CH2CH3 Solution: (i) 1 – Chloromethyl – 3 − ( 2, 2 – dimethylpropyl ) benzene (Primary benzyl halide) (ii) 1 − Bromo − 2 – ( 1 − methylpropyl ) benzene...
Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl ( primary, secondary, tertiary ), vinyl, or aryl halides
(i) CH3C(Cl)(C2H5)CH2CH3 (ii) CH3CH=C(Cl)CH2CH(CH3)2 Solution: (i) 3−Chloro−3–methylpentane (Tertiary alkyl halide) (ii) 3−Chloro−5−methylhex−2−ene (Vinyl halide)
Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl ( primary, secondary, tertiary ), vinyl, or aryl halides
(i) CH3CH(CH3)CH(Br)CH3 (ii) CH3C(C2H5)2CH2Br Solution: (i) 2 − Bromo – 3 – methylbutane (Secondary alkyl halide) (ii) 1 − Bromo − 2 − ethyl − 2 – methylbutane (Primary alkyl...
How the following conversions can be carried out?
(i) tert-Butyl bromide to isobutyl bromide (ii) Aniline to phenylisocyanide Solutions: (i) (ii)
How the following conversions can be carried out?
(i) Chloroethane to butane (ii) Benzene to diphenyl Solutions: (i) (ii)
How the following conversions can be carried out?
(i) Chlorobenzene to p-nitrophenol (ii) 2-Bromopropane to 1-bromopropane Solutions: (i) (ii)
How the following conversions can be carried out?
(i) 2-Chloropropane to 1-propanol (ii) Isopropyl alcohol to iodoform Solution: (i) (ii)
How the following conversions can be carried out?
(i) Ethyl chloride to propanoic acid (ii) But-1-ene to n-butyliodide Solution: (i) (ii)
How the following conversions can be carried out?
(i) 2-Chlorobutane to 3, 4-dimethylhexane (ii) 2-Methyl-1-propene to 2-chloro-2-methylpropane Solution: (i) (ii)
How the following conversions can be carried out?
(i) Ethanol to propanenitrile (ii) Aniline to chlorobenzene Solution: (i) (ii)
How the following conversions can be carried out?
(i) Benzene to 4-bromonitrobenzene (ii) Benzyl alcohol to 2-phenylethanoic acid Solution: (i) (ii)
How the following conversions can be carried out?
(i) 1-Bromopropane to 2-bromopropane (ii) Toluene to benzyl alcohol Solution: (i) (ii)
How the following conversions can be carried out?
(i) Propene to propan-1-ol (ii) Ethanol to but-1-yne Solution: (i) (ii) OR
Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl ( primary, secondary, tertiary ), vinyl, or aryl halides :
(i) (CH3)2CHCH(Cl)CH3 (ii) CH3CH2CH(CH3)CH(C2H5)Cl Solution: (i) 2 − Chloro − 3 – methylbutane (Secondary alkyl halide) (ii) 3 − Chloro − 4 – methyhexane (Secondary alkyl...
