Option (iii) is the answer. Aromatic alcohols are those in which the -OH group is not directly connected to the benzene ring. As a result, option C is accurate.
The process of converting alkyl halides into alcohols involves_____________. (i) addition reaction (ii) substitution reaction (iii) dehydrohalogenation reaction (iv) rearrangement reaction
Option (ii) is the answer. Since the $X^-$ group is replaced by the $OH^-$ group, the process of turning alkyl halides into alcohols may be written as: R−X→R−OH. This is a nucleophilic substitution...
CH3CH2OH can be converted into CH3CHO by ______________. (i) catalytic hydrogenation (ii) treatment with LiAlH4 (iii) treatment with pyridinium chlorochromate (iv) treatment with KMnO4
Option (iii) is the answer. Pyridinium chlorochromate (PCC), a combination of chromium trioxide, pyridine, and HCl, produces a high yield of aldehydes while preventing carboxylic acid oxidation....
What is the correct order of reactivity of alcohols in the following reaction? R—OH + HCl →(ZnCl2) R—Cl + H2O (i) 1° > 2° > 3° (ii) 1° < 2° > 3° (iii) 3° > 2° > 1° (iv) 3° > 1° > 2°
Option (iii) is the answer. The Lucas reagent is a combination of concentrated HCl and dry anhydrous $ZnCl_2$, and the reaction is known as the Lucas test. It's used to figure out whether a...
Monochlorination of toluene in sunlight followed by hydrolysis with aq. NaOH yields. (i) o-Cresol (ii) m-Cresol (iii) 2, 4-Dihydroxytoluene (iv) Benzyl alcohol
Option (iv) is the answer. Explanation: In the presence of light, halogenation follows the free radical pathway, and therefore reacts with the alkyl group to produce halo alkyl. In the presence of...
The second and third lines of progress components look like each other significantly more than they take after the primary line. Clarify why?
Solution: Because of helpless f orbital safeguarding, powerful atomic charge increments and there is a compression in the size of the third-column components. This compression in size is called...
In spite of the fact that +3 oxidation states are the trademark oxidation condition of lanthanoids cerium shows +4 oxidation state too. Why?
Solution: Ce – [Xe] 4f1 5d1 6s2. For the most part, lanthanoids lose the 5d and 6s electrons and show +3 oxidation state, yet Cerium loses the one 4f electron additionally to achieve Xenon's...
In spite of the fact that Zr has a place with 4d and Hf has a place with 5d progress series yet it is very hard to isolate them. Why?
Solution: Because of the lanthanoid compression helpless f, orbital safeguarding prompts an expansion in powerful atomic charge, which lessens the size of Hf. So the nuclear radii of both Zr and Hf...
Ionization enthalpies of Ce, Pr and Nd are higher than Th, Pa and U. Why?
Solution: Th, Pa and U, 5f electrons begin filling and they have infiltration lower than 4f electrons for Ce, Pr and Nd. Eliminating 4f electrons will be troublesome, so ionization enthalpy for Th,...
In spite of the fact that Cr3+ and Co2+ particles have similar number of unpaired electrons the attractive snapshot of Cr3+ is 3.87 B.M. furthermore, that of Co2+ is 4.87 B.M. Why?
Solution: Cr3+ has an even electron appropriation and will just have turn attractive second commitment though Co2+ has no balanced circulation of electrons so it will have an orbital attractive...
In spite of the fact that fluorine is more electronegative than oxygen, the capacity of oxygen to balance out higher oxidation states surpasses that of fluorine. Why?
Solution: Fluorine has one unpaired electron and structures a solitary bond, while Oxygen has two unpaired electrons and can shape various bonds along these lines balancing out higher oxidation...
Out of Cu2Cl2 and CuCl2, which is more steady and why?
Solution: CuCl2 is more steady in light of the fact that Cu2+ has a higher electron thickness than Cu+. Cu2+ is more modest in size, has higher powerful atomic charge and subsequently a higher...
Change components show high dissolving focuses. Why?
Solution: Change components have solid metallic bonds. Breaking those bonds becomes more diligently that implies dissolving these components will be troublesome. Thus, liquefying focuses are higher...
Why E° esteems for Mn, Ni and Zn are more negative than anticipated?
Solution: Mn2+(3d5) and Zn2+(3d10) have half-filled and filled d orbitals which give them dependability and accordingly like to remain as such and not get diminished. With respect to Ni2+(3d8), it...
For what reason does copper not supplant hydrogen from acids?
Solution: A positive decrease potential means the diminished type of Cu is more steady than hydrogen. Consequently, Cu is less receptive than hydrogen and can't dislodge it from acids.
Between sodium hydrogen carbonate and magnesium hydroxide which is a superior acid neutralizer and why?
Solution: Magnesium hydroxide is a superior acid neutralizer in light of the fact that it keeps a pH level in the stomach and insoluble in the stomach. It doesn't make stomach antacid though sodium...
What is the essential contrast among germ-killers and sanitizers?
Solution: 1. Germicides are applied to living tissues though sanitizers are applied on non-living substances 2. Germicides are antimicrobial though sanitizers are against microbial as well 3....
What is the logical clarification for the sensation of sorrow?
Solution: A chemical called Noradrenaline controls the emotional episodes. Sorrow can be caused because of the low degree of noradrenaline which hampers the sign exercises in the cerebrum.
What are analgesics?
Solution: The analgesics drug is a neurologically dynamic medication which is utilized to diminish torment. They don't have any incidental effects.
For what reason is it more secure to utilize cleanser according to the ecological perspective?
Solution: When contrasted with the cleansers are more secure to utilize which is biodegradable. It doesn't have a contaminating nature.
How does the expanding of the hydrocarbon chain of manufactured cleansers influence their biodegradability?
Solution: Lesser the expanding lesser is the non-dirtying nature of the cleanser and expanded in fanning causes the contaminating idea of the cleanser to increment.
Draw the outline showing micelle development by the accompanying cleanser. CH3(CH2)10CH2OSO3-Na+
Solution: Micelle formation of the detergent can be shown as:
Dishwashing cleansers are engineered cleansers. What is their compound nature?
Solution: Cleansers are manufactured cleansers which contain non-ionic cleansers that have a purging property. They consolidate with the soil and debasements and make them dissolvable.
Hair shampoos have a place with which class of manufactured cleanser?
Solution: Cationic cleansers are utilized in hair shampoos. eg: cetyltrimethylammonium bromide. Cationic cleansers are quarternary ammonium salts of acetic acid derivations, chlorides or bromides.
Which class of the manufactured cleansers is utilized in toothpaste?
Solution: Anionic cleansers are utilized in toothpaste to clean teeth and structure reasonable froth. Eg: sodium or ammonium lauryl sulfate
Clarify why a few times frothing is found in waterway water close to where sewage water is poured after treatment?
Solution: The froth is because of the non-biodegradable cleansers which are available in water after sewage treatment. A cleanser is a water-solvent purifying specialist which joins with...
In the event that the cleanser has high antacid substance it disturbs the skin. How could the measure of abundance soluble still up in the air? What can be the wellspring of overabundance antacid?
Solution: Overabundance of antacid can be discovered utilizing corrosive base titration. The salt that is shaped during the hydrolyses of oil during cleanser readiness might be a reason for...
What is a delicate cleanser?
Solution: They are effectively solvent which contains potassium salts of unsaturated fats as a significant part.
The two acid neutralizers and antiallergic drugs are antihistamines yet they can’t supplant one another. Clarify why?
Solution: Stomach settling agents are utilized for the treatment of corrosive in the stomach and antihistamines repress the activity of histamine in the body. The two stomach settling agents and...
Anti-inflamatory medicine is an aggravation assuaging antipyretic medication yet can be utilized to forestall coronary failure. Clarify.
Solution: Ibuprofen forestalls blood thickening in the heart as it has against blood-coagulating activity. This activity helps in forestalling coronary failure.
Which class of medications is utilized in dozing pills?
Solution: It contains sedatives as a medication which is intended for the treatment of dread, uneasiness and mental interruptions.
What is the shared trait between the anti-toxin arsphenamine and azodye?
Solution: The kind of linkage moved by the anti-microbial arsphenamine is like that of azodye. anti-toxin arsphenamine gangs – As=As-linkage which is like – N=N-linkage in azodye.
What sort of powers are associated with restricting of substrate to the dynamic site of a catalyst?
Solution: I) Van der Waal power ii) hydrogen holding iii) dipole cooperations iv) ionic securities and so forth are engaged with the limiting of substrate.
Which site of a protein is called allosteric site?
Solution: This is the site other than the dynamic site in which the medications can tie and cause its activity. They control substance responses happening in the human body.
What is the destructive impact of hyperacidity?
Solution: Hyperacidity can cause a ulcer or gastric refluxes in the stomach. The fundamental driver of hyperacidity is the discharge of corrosive in an overabundance sum.
Where are receptors found?
Solution: Receptors are found pon the cell surface layer or inside the cytoplasm. They are organic transducers.
Which sort of medications goes under antimicrobial medications?
Solution: Mostly the antimicrobial medications are utilized to treat the microbial capacities. Models like germicides, sulpha medications and anti-toxins go under this class.
What are germ-killers?
Solution: Germicides are those which are applied to the living body to forestall the development of microorganisms. It is utilized on account of cuts or wounds.
Compose the employments of medications.
Solution: Medications play a significant part in our everyday life. It fixes sicknesses. There are different sorts of medications present as tablets, syrups, salves and so on It advances and keeps...
What is the normal atomic mass of medications?
Solution: Medications have a normal atomic mass of 100-500u.
Which of the accompanying assertions are right? (a) Cationic cleansers have germicidal properties. (b) Bacteria can corrupt the cleansers containing profoundly spread chains. (c) Some engineered cleansers can give froth even in super cold water. (d) Synthetic cleansers are not cleansers.
Solution: (a, c, d) (a) Cationic cleansers are quaternary ammonium salts of amines with acetic acid derivations, chlorides or bromides as anions. These cleansers have germicidal properties. (b)...
Which of coming up next are anionic cleansers? (a) Sodium salts of sulphonated long chain liquor. (b) Ester of stearic corrosive and polyethylene glycol. (c) Quaternary ammonium salt of amine with acetic acid derivation particle. (d) Sodium salts of sulphonated long chain hydrocarbons.
Solution: (a, d) Sodium salts of sulphonated long chain liquor and sodium salts of sulphonated long chain hydrocarbons are anionic cleansers e.g., Sodium laurylsulphate CH3(CH2)10CH2OSO3–Na+ and...
Veronal and Luminal are subsidiaries of barbituric corrosive which are (i) Tranquillizers (ii) Non-narcotic analgesic (iii) Antiallergic drugs (iv) Neurologically active drugs
Solution: (a, d) Tranquilizers are neurologically dynamic medications. Veronal and luminal are subordinates of barbituric corrosive utilized as sedatives.
Amongst the accompanying antihistamine ,which are insect acids? (a)Ranitidine (b) Brompheniramine (c)Terfenadine (d)Cimetidine
Solution: (a, d) Ranitidine and cimetidine are antihistamines which are utilized as subterranean insect acids. These medication bring about arrival of lesser measure of corrosive. Brompheniramine...
Which of the accompanying mixtures are controlled as subterranean insect acids? (a) Sodium carbonate (b)Sodium Hydrogen carbonate (c)Aluminium carbonate (d)Magnism Hydroxide
Solution: (b,d) Sodium Hydrogen carbonate and Magnism Hydroxide ,both are gentle alkalies ,are utilized as subterranean insect acids.
Which of the accompanying assertions are inaccurate with regards to penicillin? (a) An antibacterial organism. (b) Ampicillin is its engineered adjustment. (c) It has bacteriostatic impact. (d) It is a wide range anti-infection.
Solution: (c, d) Penicillin annihilates microbes by obliterating the cell mass of the microorganism or kill the microscopic organisms along these lines, it has bacteriocidal impact. Penicillin has a...
Which of coming up next are antidepressants? (a) Iproniazid (b) Phenelzine (c) Equanil (d) Salvarsan
Solution: (a, b, c) (a) Iproniazid is a hydrazine drug utilized as an upper. (b) Phenelzine is otherwise called Nardil. It is utilized in the treatment of significant burdensome problem. (c) Equanil...
Which of coming up next are sulpha drugs? (a) Sulphapyridine (b) Prontosil (c) Salvarsan (d) Nardil
Solution: (a, b) (a) Sulphapyridine is a sulphonamide antibacterial medication. (b) Prontosil is additionally called sulphamidochrysoidine. (c) Salvarsan is arsenic based antibacterial medication....
Which of the accompanying assertions are right with regards to barbiturates? (a) Hypnotics or rest creating specialists. (b) These are sedatives. (c) Non-opiate analgesics. (d) Pain diminishing without upsetting the sensory system.
Solution: (a, b) Barbiturates are sedatives which are utilized as hypnotics or rest instigating specialists.
Mixtures with clean properties are (a) CHCl, (b) CHI3 (c) Boric corrosive (d) 0.3 ppm watery arrangement of Cl2
Solution: (b, c) (a) CHCl3 (chloroform) was utilized as a sedation in medical procedure yet presently it is utilized in the creation of the Freon refrigerant R-22. (b) Iodoform (CFH3) produces...
Which of coming up next are not utilized as food additives? (a) Table salt (b) Sodium hydrogen carbonate (c) Cane sugar (d) Benzoic corrosive
Solution: (b, d) Table salt and raw sweetener are utilized, as food additives while sodium hydrogen carbonate and benzoic corrosive are not utilized as food additives.
Which of the accompanying assertions are erroneous with regards to receptor proteins? (a) Majority of receptor proteins are installed in the cell layers. (b) The dynamic site of receptor proteins opens within locale of the cell. (c) Chemical couriers are gotten at the limiting locales of receptor proteins. (d) Shape of receptor doesn’t change during connection of courier.
Solution: (b, d) Receptor proteins are implanted in the cell film and their dynamic destinations project outside area of the cell layer. State of the receptor changes during the connection of...
Which of the accompanying won’t improve dietary benefit of food? (a) Minerals (b) Artificial sugars (c) Vitamins (d) Amino acids
Solution: (b) Artificial sugars are non-caloric substitutes for sugar. They are frequently strongly more sweet than sugar however don't upgrade healthy benefit of food. Nutrients and minerals are...
Which of the accompanying synthetic can be added for improving of food things at cooking temperature and doesn’t give calories? (a) Sucrose (b) Glucose (c) Aspartame (d) Sucralose
Solution: (d) Sucralose is a fake improving specialist which is multiple times better than sucrose and doesn't give calories.
Which of the accompanying assertion isn’t correct with regards to chemical inhibitors? (a) Inhibit the synergist movement of the chemical. (b) Prevent the limiting of substrate. (c) Generally, a solid covalent bond is shaped between an inhibitor and a compound. (d) Inhibitors can be cutthroat or non-serious.
Solution: (c) Inhibitors are synthetic substances which will in general lessen the action of a specific chemical. For the most part, a powerless bond, for example, H-holding, van der Waals...
Which of coming up next isn’t an objective particle for drug work in body? (a) Carbohydrates (b) Lipids (c) Vitamins (d) Proteins
Solution: (c) Drugs generally associate with biomolecules like starches, lipids, proteins and nucleic acids. These are called drug targets. Nutrients are not an objective atom for drug work in...
Polyethyleneglycols are utilized in the readiness of which kind of cleansers? (a) Cationic cleansers . (b) Anionic cleansers (c) Non-ionic cleansers (d) Soaps
Solution: (c) Polyethyleneglycols are utilized in the planning of non-ionic cleansers.
Which of coming up next is an illustration of fluid dishwashing cleanser?
Solution: (b) Liquid dishwashing cleansers are non-ionic cleansers.
Glycerol is added to cleanser. It capacities (a) as a filler (b) to increment leathering (c) to forestall quick drying (d) to make cleanser granules
Solution: (c) Glycerol is added to shaving cleanser to forestall quick drying while to upgrade the leathering property of cleanser, a gum called rosin is added to them. It structures sodium rosinate...
Which of the accompanying improves washed property of cleanser? (a) Sodium carbonate (b) Sodium rosinate (c) Sodium stearate (d) Trisodium phosphate
Solution: (b) Shaving cleansers contain glycerol to forestall quick drying. A gum called rosin is included these cleansers which structures sodium rosinate which upgrades washed property of...
A gum rosin added to cleanser to make it foam well. Bithional is added to cleansers to bestow germ-free properties to cleanser. Equanil is (a) fake sugar (b) sedative (c) antihistamine (d) antifertility medication
Solution: (b) Equanil is a sedative utilized in controlling sadness and hypertension.
Compound which is added to cleanser to confer disinfectant properties is (a) sodium laurylsulphate (b) sodium dodecylbenzenesulphonate (c) rosin (d) bithional
Solution: (d) All cleansers are made by bubbling fats or oils with reasonable hydroxide. Varieties are made by adding distinctive natural substances. Sodium laurylsulphate and sodium...
The compound that causes general upper activity on the focal sensory system has a place with the class of (a) analgesics (b) sedatives (c) opiate analgesics (d) antihistamines
Solution: (b) The compound that causes general stimulant activity on the focal sensory system has a place with the class of sedatives.
A limited range anti-microbial is dynamic against (a) gram positive or gram negative microbes (b) gram negative microscopic organisms as it were (c) single creature or one sickness (d) both gram positive and gram negative microbes
Solution: (a) A tight range anti-microbial is dynamic against gram positive or gram negative microorganisms.
Salvarsan is arsenic containing drug which was first utilized for the treatment of (a) syphilis (b) typhoid (c) meningitis (d) loose bowels
Solution: (a) Salvarsan is arsenic containing drug which was first utilized for treatment of syphilis. Syphilis is an intense and ongoing contaminations sickness brought about by the bacterium...
Which of the accompanying assertion is right? (a) Some sedatives work by hindering the compounds which catalyze the debasement of noradrenaline. (b) Tranquilizers are opiate drugs. (c) Tranquilizers are substance intensifies that don’t influence the message move from nerve to receptor. (d) Tranquilizers are substance intensifies that can calm torment and fever.
Solution: (a) Tranquilizers are neurologically dynamic medications. A few sedatives are antidepressants and the capacities by hindering the proteins which catalyze the corruption of noradrenaline....
The most valuable order of medications for restorative scientific experts is (a) based on synthetic design (b) based on drug activity (c) based on sub-atomic targets (d) based on pharmacological impact
Solution: (c) The most valuable grouping of medications for therapeutic physicists. It is based on sub-atomic targets. Target particles are typically biomolecules like carbs, lipids, proteins and...
Which proclamation about ibuprofen isn’t correct? (a) Aspirin has a place with opiate analgesics. (b) It is powerful in diminishing torment. (c) It has antiblood thickening activity. (d) It is a neurologically dynamic medication.
Solution: (a) Aspirin restrains the combination of mixtures known as prostaglandins which invigorate irritation in the tissues and cause torment. Thus, it is viable in easing torment. Ibuprofen has...
Which is the right assertion about anti-conception medication pills? (a) Contain estrogen as it were (b) Contain progesterone as it were (c) Contain a combination of estrogen and progesterone subsidiaries (d) Progesterone upgrades ovulation
Solution: (c) Birth control pills contain a combination of estrogen and progesterone subsidiaries. Both of these are sex chemicals. Progesterone stifles ovulation and estrogen control the feminine...
Which of the accompanying assertion isn’t right? (a) Some germ-killers can be added to cleansers. (b) Dilute arrangements of certain sanitizers can be utilized as germ-free. (c) Disinfectants are antimicrobial medications. (d) Antiseptic prescriptions can be ingested.
Solution: (d) Antiseptics are applied to the living tissues like injuries, cuts and unhealthy skin surfaces. Disinfectant prescriptions, for example, anti-microbials can't be ingested.
What is the structure and IUPAC name of glycerol?
Glycerol, commonly known as glycerin, is a triol molecule found in both plant and animal lipids. It's a substance that's utilised in dermatological treatments. It has mostly been utilised as a...
Write the IUPAC name of the following compounds.
(A)The compound's IUPAC name is 3-Ethyl-5-methyl hexane-2,4-diol. (B) The compound's IUPAC name is 1-Methoxy-3-nitrocyclohexane
Write the IUPAC name of the compound given below.
The compound's IUPAC name is 3-Methylpent-2-ene-1,2-diol. In the structure shown, the longest carbon atom chain is 5. Except for one double bond between C2 and C3 atoms, all carbon atom bonds are...
Name the factors responsible for the solubility of alcohols in water.
The following factors influence the solubility of alcohols in water: (i)Hydrogen bonding (ii)The size of the alkyl or aryl groups is the second factor to consider. (iii)The molecular mass of...
What is denatured alcohol?
Alcohols used for drinking are rendered unsuitable for human consumption by combining them with copper sulphate and pyridine, which give the liquid a yellow colour and a terrible odour,...
Suggest a reagent for the following conversion.
The oxidation of secondary alcohol into a ketone is seen in the chemical process above. Using oxidising agents such as chromic anhydride (CrO3), Pyridinium chlorochromate (PCC), and others, this is...
Out of 2-chloroethanol and ethanol which is more acidic and why?
Because of the presence of chlorine, which is an electron-withdrawing group, 2-chloroethanol is more acidic. The electron density in the –O-H bond decreases as a result of the negative inductive...
Suggest a reagent for conversion of ethanol to ethanal.
As reagents, PCC or Pyridinium chlorochromate might be employed. The oxidation of primary alcohol to an aldehyde is seen in the diagram above.
Suggest a reagent for conversion of ethanol to ethanoic acid.
For the afore mentioned reaction, acidified KMnO4 might be employed as a reagent. The oxidation of primary alcohol to a carboxylic acid is seen in the diagram above.
Out of o-nitrophenol and p-nitrophenol, which is more volatile? Explain.
O-nitrophenol is more volatile due to intramolecular hydrogen bonding between the NO2 and OH groups.
When phenol is treated with bromine water, a white precipitate is obtained. Give the structure and the name of the compound formed.
2,4,6-tribromophenol is the name of the chemical produced in this process. The compound's structure is as follows:
Arrange the following compounds in increasing order of acidity and give a suitable explanation. Phenol, o-nitrophenol, o-cresol
o-cresol < Phenol < o-nitrophenol. NO2 o-nitrophenol becomes more acidic due to the presence of the electron-withdrawing group. The electron-releasing group in remaining reduces the acidic...
Alcohols react with active metals e.g. Na, K etc. to give corresponding alkoxides. Write down the decreasing order of reactivity of sodium metal towards primary, secondary and tertiary alcohols
The following is a list of sodium metal's reactivity to alcohols in decreasing order: Primary alcohols are followed by secondary alcohols and finally tertiary alcohols. Because of two factors,...
What happens when benzene diazonium chloride is heated with water?
When benzene diazonium chloride is heated with water, it produces phenol as well as nitrogen gas and hydrochloric acid as by-products.
Arrange the following compounds in decreasing order of acidity. H2O, ROH, HC ≡ CH
The acidity of the following substances is listed in decreasing order: H2O > ROH > HC ≡ CH Because the carbon atoms here are sp hybridised, the electron density on the carbon atom is greater,...
Name the enzymes and write the reactions involved in the preparation of ethanol from sucrose by fermentation.
The enzymes involved in the fermentation of sucrose to produce ethanol are known as invertase and zymase. Sucrose is converted to glucose and fructose by invertase. The glucose and fructose are then...
How can propane-2-one be converted into tert- butyl alcohol?
Propane-2-one is hydrolyzed after being treated with CH3MgBr in the presence of dry ether (Grignard reagent).
Explain why the OH group in phenols is more strongly held as compared to OH group in alcohols
The phenol –OH group is immediately linked to the benzene ring's sp2hybridized carbon atom. The carbon-oxygen bond length in phenol is shorter than that in alkyl alcohol, which is related to the...
Explain why nucleophilic substitution reactions are not very common in phenols.
The ortho- and para-positions of the benzene ring become electron-rich as a result of the resonance, activating it for electrophilic substitution reactions. As a result, nucleophilic substitution...
Preparation of alcohols from alkenes involves the electrophilic attack on an alkene carbon atom. Explain its mechanism.
Step 1: Alkene protonation and production of a carbocation Step 2: Water's nucleophilic assault Step 3: Deprotonation occurs, resulting in the formation of alcohol. H30+ is now...
Explain why is O=C=O nonpolar while R—O—R is polar.
The dipole moments of the two C=O bonds are exactly equal and opposite, making O=C=O nonpolar. As a result, they cancel each other out, resulting in a net dipole moment of zero for O=C=O. Because...
Why is the reactivity of all the three classes of alcohols with conc. HCl and ZnCl2 (Lucas reagent) different?
This is due to the alkyl group's steric barrier and the carbocation's stability. Because the 1° carbocation is the least stable, primary alcohol has no reaction at ambient temperature. At ambient...
Write steps to carry out the conversion of phenol to aspirin.
Acetylsalicylic acid is another name for aspirin. The phenoxide ion is created by treating phenol with NaOH. The phenoxide ion is subsequently electrophilically substituted with CO2 to produce...
Nitration is an example of aromatic electrophilic substitution and its rate depends upon the group already present in the benzene ring. Out of benzene and phenol, which one is more easily nitrated and why?
The presence of the hydroxyl group in phenol causes it to be more nitrated. The ortho- and para-positions in the benzene ring become electron-rich due to the resonance effect induced by the –OH...
In Kolbe’s reaction, instead of phenol, phenoxide ion is treated with carbon dioxide. Why?
Because phenoxide ion is more reactive towards electrophilic aromatic substitution than phenol, phenoxide ion is treated with carbon dioxide (a weak electrophile) in Kolbe's process.
The dipole moment of phenol is smaller than that of methanol. Why?
The dipole moment of phenol is smaller than that of methanol due to the electron-withdrawing effect of the phenyl ring. Due to the resonance, the polarity of the C-O bond in phenol decreases.
Ethers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can’t be prepared by this method. Explain.
Williamson synthesis, in which an alkyl halide is reacted with sodium alkoxide, can be used to make ethers. This technique cannot be used to make di-tert-butyl ether because elimination takes...
Why is the C—O—H bond angle in alcohols slightly less than the tetrahedral angle whereas the C—O—C bond angle in ether is slightly greater?
Due to repulsion between the unshared pair of electrons or the lone pair of electrons on the oxygen atom, the C—O—H bond angle in alcohols is somewhat smaller than the tetrahedral angle.
Explain why low molecular mass alcohols are soluble in water.
This is due to the presence of intermolecular hydrogen bonding between alcohol molecules due to the presence of the OH group. The impact of the polar character of the –OH group of alcohol is...
Explain why p-nitrophenol is more acidic than phenol.
Because of the presence of an electron-withdrawing group, the -NO2 group, which improves the acidic strength of the molecule by stabilising the phenoxide ion, para-nitrophenol is more acidic than...
Explain why alcohols and ethers of comparable molecular mass have different boiling points?
Because alcohols contain intermolecular hydrogen bonding, their boiling temperatures differ from those of ethers of comparable molecular mass.
The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
Reason: (i) The carbon-oxygen bond obtains a partial double bond character due to the resonance. As a result, the carbon-oxygen bond in phenol shrinks in size. ii) Oxygen is directly connected to a...
Define environmental chemistry.
The study of biological and chemical processes that occur in nature is referred to as environmental chemistry. It also investigates the chemical species' reactions, origins, impacts, and...
Depict the galvanic cell in which the cell reaction is Cu + 2Ag+→ 2Ag + Cu2+
In the cell reaction Cu + 2Ag+→ 2Ag + Cu2+, Anode is the oxidation half-cell Reaction - Cu → Cu2+ + 2e- Cathode is the reduction half-cell Reaction - 2Ag+ + 2e- →2Ag
Why are solids incompressible?
The intermolecular distance is less within the molecules of a solid and repulsive force occurs between the electron clouds which makes the solids incompressible.
The percentage of empty space in a body centred cubic arrangement is ________.
(i) 74 (ii) 68 (iii) 32 (iv) 26 Correct Answer: (iii) 32 Explanation: Calculation – Empty space in a body-centered arrangement = 100 – 68 Empty space in a body-centered arrangement =...
Which of the following is true about the value of the refractive index of quartz glass?
(i) Same in all directions (ii) Different in different directions (iii) Cannot be measured (iv) Always zero Correct Answer: (i) Same in all directions Explanation: As the amorphous...
Which of the following arrangements shows the schematic alignment of magnetic moments of antiferromagnetic substances?
Correct Answer: Option (d) Explanation: These are the factors in which the electron spins are associated with magnetic atoms in certain crystallographic areas and are directed at each other in such...
Which of the following is an amorphous solid?
(i) Graphite (C) (ii) Quartz glass (SiO2) (iii) Chrome alum (iv) Silicon carbide (SiC) Correct Answer: (ii) Quartz glass (SiO2) Explanation: Solids are considered as an amorphous when they...
Which of the following is not a characteristic of a crystalline solid?
(i) Definite and characteristic heat of fusion (ii) Isotropic nature (iii) A regular periodically repeated pattern of arrangement of constituent particles in the entire crystal (iv) A true solid...
Which of the following conditions favours the existence of a substance in the solid state?
(i) High temperature (ii) Low temperature (iii) High thermal energy (iv) Weak cohesive forces Correct Answer: (ii) Low temperature Explanation: At the low temperatures, the substances...
The element which exists in a liquid state for a wide range of temperature and can be used for measuring high temperature is (i) B (ii) Al (iii) Ga (iv) In
The solution is option (iii).
Hydrogen bonds are formed in many compounds e.g., H2O, HF, NH3. The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of the above compounds is : (i) HF > H2O > NH3 (ii) H2O > HF > NH3 (iii) NH3 > HF > H2O (iv) NH3 > H2O > HF
Solution: Option (ii) is the answer.
Explain what is observed when
(i) when a beam of light is passed through a colloidal sol,
(ii) an electrolyte NaCl is added to hydrated to ferric oxide sol, and
(iii) electric current is passed through a colloidal sol?
Solution:- (i) When a beam of light is transmitted through a colloidal solution, light scattering is detected. This phenomenon is called the Tyndall effect. The path of the beam is...
Define emulsions. What are their different types? Give examples of each type.
The colloidal solution in which both the dispersed phase and the dispersion medium are liquids is known as an emulsion. Emulsions are divided into two categories: (a) Oil in water type: The...
The two strands in DNA are not identical but are complementary. Explain.
The hydrogen bond maintains the two strands of DNA together between specified pairs of bases in the helical helix. Adenine and thymine make a hydrogen link, while cytosine and guanine form a...
Predict the products of electrolysis in each of the following:
(iii) A dilute solution of 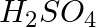 with platinum electrodes.
with platinum electrodes.
(iv) An aqueous solution of 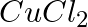 with platinum electrodes.
with platinum electrodes.
Solution: (iii) At the cathode, the following reduction reaction occurs to produce $H_{2}$ gas. $$ H{(a q)}^{+}+e^{-} \rightarrow \frac{1}{2} H_{2(g)}$$At the anode, the following processes are...
Predict the products of electrolysis in each of the following:
(i) An aqueous solution of 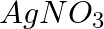 with silver electrodes. (ii) An aqueous solution of
with silver electrodes. (ii) An aqueous solution of 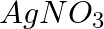 with platinum electrodes.
with platinum electrodes.
Solution: (i) At the cathode: The following reduction reactions are in competition with one another for space at the cathode.$$A g_{(a q)}^{+}+e^{-} \rightarrow A g_{(s)} ; E ^{0}=0.80 V$$$$H_{(a...
Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(v) 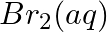 and
and 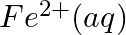
Solution: When it comes to electrochemistry, the term "standard electrode potential" refers to the value obtained by measuring the standard emf of a cell in which molecular hydrogen under standard...
Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(iii) 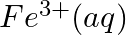 and
and 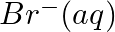
(iv) 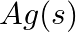 and
and 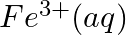
Solution: When it comes to electrochemistry, the term "standard electrode potential" refers to the value obtained by measuring the standard emf of a cell in which molecular hydrogen under standard...
Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(i) 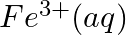 and
and 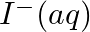
(ii) 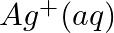 and
and 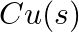
Solution: When it comes to electrochemistry, the term "standard electrode potential" refers to the value obtained by measuring the standard emf of a cell in which molecular hydrogen under standard...
Three electrolytic cells A, B,C containing solutions of 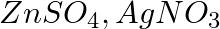 and
and 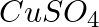 , respectively are connected in series. A steady current of
, respectively are connected in series. A steady current of 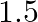 amperes was passed through them until
amperes was passed through them until 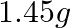 of silver was deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited?
of silver was deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited?
According to the information given in the question the reaction will be,$$A g_{(a q)}^{+}+e^{-} \rightarrow A g_{(s)}$$i.e., $108 g$ of $Ag$ is deposited by $96487 C$.Therefore, $1.45 g$ of $Ag$ is...
A solution of 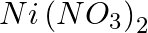 is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Solution: As a result of the reaction, it is $$N i^{2+}+2 e^{-} \rightarrow N i_{(s)}+e^{-}$$ Nickel deposited by $2 \times 96487 C =58.71 g$ Therefore, nickel deposited by $6000 C =\frac{58.71...
How much electricity is required in coulomb for the oxidation of
(i) 1 mol of  to
to 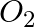 ?
?
(ii) 1 mol of FeO to 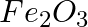 ?
?
Solution: (i) From given data, we write the equations that can be derived$$H_{2} O \rightarrow H_{2}+\frac{1}{2} O_{2} $$ We can say that : $$ O^{2-} \rightarrow \frac{1}{2} O_{2}+2...
How much electricity in terms of Faraday is required to produce
(i) 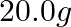 of
of 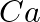 from molten
from molten 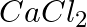 ?
?
(ii) 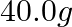 of
of 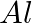 from molten
from molten 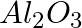 ?
?
Solution: (i) From given data, we write the equation of cell, $$Ca ^{2+}+2 e^{-} \rightarrow Ca$$ Evaluating the value of electricity in terms of Faraday we have, Electricity required to produce $40...
How much charge is required for the following reductions:
(i) 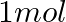 of
of 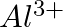 to
to 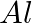 ?
?
(ii) 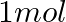 of
of 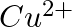 to
to 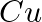 ?
?
(iii) 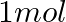 of
of 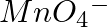 to
to 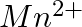 ?
?
Solution: As we know that, 1 mole of electron has a charge of 1 faraday (i) $A l^{3+}+3 e^{-} \rightarrow A l$Required charge $=3 F$$=3 \times 96487 C$$=289461 C$(ii) $C u^{2+}+2 e^{-} \rightarrow C...
Conductivity of 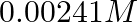 acetic acid is
acetic acid is 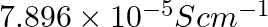 . Calculate its molar conductivity. If
. Calculate its molar conductivity. If 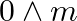 for acetic acid is
for acetic acid is 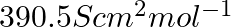 , what is its dissociation constant?
, what is its dissociation constant?
Solution: Given, $K =7.896 \times 10^{-5} S m ^{-1} c$ $$=0.00241 mol L ^{-1}$$As we know, molar conductivity, $\Lambda_{m}=\frac{k}{c}$ Substituting the value of known parameters we...
The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below. Calculate Λm for all concentrations and draw a plot between Λm and c½. Find the value of 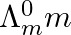
Solution: Evaluating the value of Λm for each case, Given,$$K =1.237 \times 10^{-2} S m -1, c =0.001 M$$Then, $K =1.237 \times 10^{-4} S cm ^{-1}, c ^{1 / 2}=0.0316 M ^{1 /...
The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 Ω. What is the cell constant if the conductivity of 0.001M KCl solution at 298 K is 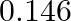

Solution: Given,Conductivity, $k =0.146 \times 10^{-3} S cm -1$Resistance, $R =1500 \Omega$Concept : We know the formula is, $$ \text { Cell constant }= k \times R$$ Calculation: Substuting the...
The conductivity of 0.20 M solution of KCl at 298 K is 0.0248  . Calculate its molar conductivity.
. Calculate its molar conductivity.
Solution: Given, $K =0.0248 S cm ^{-1} C$$$c =0.20 M$$ Concept: We know that Molar conductivity, $\Lambda_{m}=\frac{k \times 1000}{c}$ Calculation: Substituting the value of known parameter,...
Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
Solution: Conductivity: The conductivity of a solution is defined as the conductance of a solution with a length of 1 cm and an area of cross-section of 1 sq. cm and a cross-sectional area of 1 sq....
In the button cells widely used in watches and other devices, the following reaction takes place. Determine ∆rGJ and EJ for the reaction.
Solution: Given, $E_0 = 1.104 V$ We know that, $$\Delta_{r} G^{\Theta}=-n F E^{\Theta}$$ Thus substituting the value of parmeters. = −2 × 96487 × 1.04 = −213043.296 J = −213.04...
Write the Nernst equation and emf of the following cells at 298 K: (i) 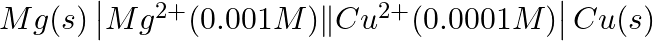
(ii) 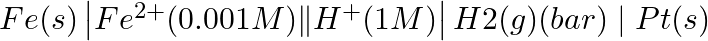
Solution: Nernst equation in electrochemistry is a relationship between the reduction potential of a reaction (either a half-cell or full-cell reaction) and various parameters such as the standard...
Write the Nernst equation and emf of the following cells at 298 K: (iii) 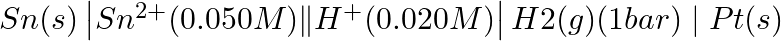
(iv) 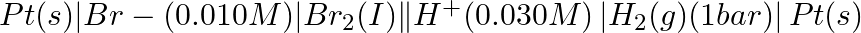
Solution: Nernst equation in electrochemistry is a relationship between the reduction potential of a reaction (either a half-cell or full-cell reaction) and various parameters such as the standard...
Calculate the standard cell potentials of galvanic cell in which the following reactions take place: (i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd (ii) Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s) Calculate the ∆rGJ and equilibrium constant of the reactions.
Solution: (i) Given: $E_{C r^{3+} / C r}^{\Theta}=0.74 V$ $$E_{C d^{2+} / C d}^{\Theta}=-0.40 V$$The galvanic cell of the given reaction is represented as :$$C r_{(s)}\left|C r_{(a q)}^{3+} | C d_{a...
Depict the galvanic cell in which the reaction 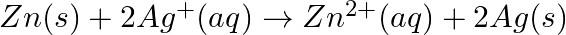 takes place. Further show: (i) Which of the electrode is negatively charged? (ii) The carriers of the current in the cell. (iii) Individual reaction at each electrode.
takes place. Further show: (i) Which of the electrode is negatively charged? (ii) The carriers of the current in the cell. (iii) Individual reaction at each electrode.
Solution: It is an electrochemical cell named after the inventors Luigi Galvani and Alessandro Volta, respectively, in which an electric current is generated through spontaneous reactions. The...
Given the standard electrode potentials,
K+/K = –2.93V
Ag+/Ag = 0.80V,
Hg2+/Hg = 0.79V
Mg2+/Mg = –2.37 V,
Cr3+/Cr = – 0.74V
Solution: As the reduction potential is reduced, the reducing power increases proportionally. The following are the standard electrode potentials in ascending order (increasing order): K+/K <...
Arrange the following metals in the order in which they displace each other from the solution of their salts. Al, Cu, Fe, Mg, and Zn
Solution: According to their reactivity, the following metals replaces in the following order: magnesium, aluminum, zinc, iron, and copper. Magnesium > Aluminum > Zinc > Copper
Give a plausible explanation for this: Why are aromatic amines weaker bases than aliphatic amines?
Because of the – R impact of the benzene ring, aromatic amines have lower availability of N atoms. As a result, the electrons on the N – atom in aromatic amines are difficult to transfer. As a...
Give a plausible explanation for each of the following:
(i) Why are alcohols more acidic than amines of comparable molecular masses?
(ii) Why do tertiary amines have lower boiling points than primary amines?
(i) protonation of amines gives amide ion. Similarly, alcohol gives away a proton which results in alkoxide ions. In an amide ion, the negative charge is on the N-atom, whereas in an alkoxide ion,...
Write the reactions of
(i) Aromatic with nitrous acid.
(ii) Aliphatic primary amines with nitrous acid.
(i) When aromatic amine reacts with nitrous acid (which is generated in situ from NaNO2) and a mineral acid like phosphoric acid, it produces nitrous oxide. (ii) When aliphatic primary amines react...
Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
The Gabriel phthalimide synthesis is most commonly employed to make aliphatic primary amines. Nucleophilic substitution (SN2) of alkyl halides by the anion generated by the phthalimide is an example...
Complete the following reactions:
(i) (ii) (iii) Answer: (i) (ii) (iii)
Complete the following reactions:
(i) (ii) Answer: (i) (ii)
Complete the following reactions:
An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Bromine and KOH forms a compound ‘C’ of molecular formula C6H7N. Write the structures and IUPAC names of compounds A, B, and C.
Compound ‘C,' with the molecular formula C6H7N, is created by heating compound ‘B' with Br2 and KOH, according to the formula. This is a decomposition of Hoffmann bromamide. As a result, compound B...
Give the structures of A, B, and C in the following reactions:
(i) (ii) Answer: (i) (ii)
Give the structures of A, B, and C in the following reactions:
(i) (ii) Answer: (i) (ii)
Give the structures of A, B, and C in the following reactions:
Accomplish the following conversions:
(i) Benzyl chloride to 2 – phenylethanamine
(ii) Chlorobenzene to p – chloroaniline
(i) (ii)
Accomplish the following conversions:
(i) Benzoic acid to aniline
(ii) Aniline to 2, 4 ,6 – tribromofluorobenzene
(i) Benzoic acid to aniline <br>(ii) Aniline to 2, 4 ,6 – tribromofluorobenzene (i) (ii)
Accomplish the following conversions:
(i) Nitrobenzene to benzoic acid
(ii) Benzene to m – bromophenol
Write short notes on the following:
(i) Ammonolysis
(ii) Acetylation
(iii) Gabriel phthalimide synthesis.
(i) When an alkyl or benzyl halide reacts with an ethanolic solution of ammonia, the halogen atom is replaced by an amino ( – NH2) group in a nucleophilic substitution process. Ammonolysis is the...
Write short notes on the following:
(i) Hofmann’s bromamide reaction
(ii) Coupling reaction
(i) A primary amine with one carbon atom less than the original amide is formed when an amide is treated with bromine in an aqueous or ethanolic solution of sodium hydroxide. The Hoffmann bromamide...
Write short notes on the following:
(i) Carbylamine reaction(ii) Diazotisation
(i) The carbylamine reaction is a test for determining the presence of primary amines. Carbylamines (or isocyanides) are produced d when aliphatic and aromatic primary amines are heated with...
Describe a method for the identification of primary, secondary, and tertiary amines. Also, write chemical equations of the reactions involved.
Hinsberg's test can be used to identify and discriminate primary, secondary, and tertiary amines. The amines are allowed to react with Hinsberg's reagent, benzene sulphonyl chloride(C6H5SO2 Cl)....
How will you convert:
(i) Nitromethane into dimethylamine (ii) Propanoic acid into ethanoic acid
(i) (ii)
How will you convert:
(i) Ethanoic acid into propanoic acid (ii) Methanamine into ethanamine
(i) (ii)
How will you convert:
(i) Methanol to ethanoic acid (ii) Ethanamine into methanamine
(i) (ii)
How will you convert:
(i) Ethanoic acid into methanamine (ii) Hexanenitrile into 1–aminopentane
Arrange the following:
(i) In decreasing order of basic strength in the gas phase: ![Rendered by QuickLaTeX.com \[{{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{N}{{\mathbf{H}}_{\mathbf{2}}},\text{ }{{\left( {{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}} \right)}_{\mathbf{2}}}\mathbf{NH},\text{ }{{\left( {{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}} \right)}_{\mathbf{3}}}\mathbf{N}\text{ }\mathbf{and}\text{ }\mathbf{N}{{\mathbf{H}}_{\mathbf{3}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7543ab20599101d93b093dbe8157db71_l3.png)
(ii) In increasing order of boiling point: ![Rendered by QuickLaTeX.com \[{{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{OH},\text{ }{{\left( \mathbf{C}{{\mathbf{H}}_{\mathbf{3}}} \right)}_{\mathbf{2}}}\mathbf{NH},\text{ }{{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{N}{{\mathbf{H}}_{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7401558baa4042733e47b502dc9be068_l3.png)
(iii) In increasing order of solubility in water: ![Rendered by QuickLaTeX.com \[{{\mathbf{C}}_{\mathbf{6}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{N}{{\mathbf{H}}_{\mathbf{2}}},\text{ }{{\left( {{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}} \right)}_{\mathbf{2}}}\mathbf{NH},\text{ }{{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{N}{{\mathbf{H}}_{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ca2c31e232cd6bffda273a89d9fc4d7d_l3.png)
(i) There is no solvation effect in the gas phase. As a result, the + I impact is primarily responsible for the fundamental strength. The firmer the base, the larger the +I impact. In addition, the...
Arrange the following in increasing order of basic strength:
(a) Aniline, p-nitroaniline and p-toluidine
![Rendered by QuickLaTeX.com \[\left( \mathbf{b} \right)\text{ }{{\mathbf{C}}_{\mathbf{6}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{N}{{\mathbf{H}}_{\mathbf{2}}},\text{ }{{\mathbf{C}}_{\mathbf{6}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{NHC}{{\mathbf{H}}_{\mathbf{3}}},\text{ }{{\mathbf{C}}_{\mathbf{6}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{C}{{\mathbf{H}}_{\mathbf{2}}}\mathbf{N}{{\mathbf{H}}_{\mathbf{2}}}.\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c77fbba6b41e37702bf58112d1373bda_l3.png)
(a) The presence of the electron-donating – CH3 group in p – toluidine boosts the electron density on the N-atom. As a result, p – toluidine has higher basicity than aniline. In the case of p...
Arrange the following:
(i) In decreasing order of the pKb values:C2H5NH2,C6H5NHCH3,(C2H5)2NH,C6H5NH2
(ii) In increasing order of basic strength: C6H5NH2,C6H5N(CH3)2,(C2H5)2NH,CH3NH2
(i) C2 H5 NH2 consists of one –C2 H5 group. (C2 H5 )2NH consists of two –C2 H5 groups. As a result, the +I effect in ( C2H5)2NH is greater than that in...
Account for the following:
(i) Aniline does not undergo Friedel-Crafts reaction.
(ii) Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(iii) Gabriel phthalimide synthesis is preferred for synthesizing primary amines
(i) Aniline does not undergo Friedel – Crafts reaction. In the presence of AlCl 3, the Friedel–Crafts reaction is carried out. However, we already know that AlCl 3 is acidic, whereas aniline is not....
Account for the following:
(i) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
(ii) Although the amino group is o– and p– directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
(i) Methylamine in the water when made to react with ferric chloride, precipitates hydrated ferric oxide. Water is less basic than methylamine due to the presence of the – CH3 group and the + I...
Account for the following:
(i) p Kb of aniline is more than that of methylamine.(ii) Ethylamine is soluble in water whereas aniline is not. (i) p Kb of methylamine is lesser than that of aniline : The electrons accessible on...
Give one chemical test to distinguish between the following pairs of 2:
(i) Ethylamine and aniline (ii) Aniline and benzylamine(iii) Aniline and N-methylaniline. (i) azo–dye test can distinguish aniline & Ethylamine. When aromatic amines react...
Give one chemical test to distinguish between the following pairs of compounds:
(i) Methylamine and dimethylamine (ii) Secondary and tertiary amines\ (i) dimethylamine & Methylaminecan be made notable by the carbylamine test. When aliphatic and aromatic primary amines...
Write IUPAC names of the following compounds and classify them into primary, secondary, and tertiary amines: (i) C6H5NHCH3 (ii) (CH3CH2)2NCH3 (iii) m–BrC6H4NH2
(i) N – Methyl benzamine or N – methylaniline ( 20 amine ) (ii) N – Ethyl – N – methyl ethanamine ( 30 amine ) (iii) 3 – Bromobenzenamine or 3 – bromoaniline ( 10 amine...
Write IUPAC names of the following compounds and classify them into primary, secondary, and tertiary amines.
(i) CH3 NH CH ( CH3 )2 (i) ( CH3 )3 CNH 2 (i) N – Methyl – 2 – methyl ethanamine ( 20 amine ) (ii) 2 – Methylpropan – 2 – amine ( 10 amine...
Write IUPAC names of the following compounds and classify them into primary, secondary, and tertiary amines.
(i) ( CH3 )2 CH NH 2 (ii) CH3 ( CH2 )2 NH 2
If a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL of water at 37°C, calculate the osmotic pressure in Pascal exerted by it?
It is given that: Volume of water $(\mathrm{V})=450 \mathrm{~mL}=0.45 \mathrm{~L}$ Temperature (T) $=37+273=310 \mathrm{~K}$ moles of the polymer, $\mathrm{n}=\frac{1}{185000} \mathrm{~mol}$ We know...
How much of sucrose is to be added to 500 g of water such that it boils at 100°C if the molar elevation constant for water is 0.52 K kg mol-1 and the boiling point of water at 750 mm Hg is 99.63°C?
Elevation in boiling point $\Delta T_{b}=(100+273)-(99.63+273)$ $=0.37 \mathrm{~K}$ Mass of water, $w_{1}=500 \mathrm{~g}$ Molar mass of sucrose $\left(\mathrm{C}_{12} \mathrm{H}_{22}...
Find the vapor pressure of water and its relative lowering in the solution which is 50 g of urea (NH2CONH2) dissolved in 850 g of water. (Vapor pressure of pure water at 298 K is 23.8 mm Hg)
As we know that vapour pressure of water, $P_{1}^{\infty}=23.8 \mathrm{~mm}$ of $\mathrm{Hg}$ Weight of water, $w_{1}=850 \mathrm{~g}$ Weight of urea, $w_{2}=50 \mathrm{~g}$ Molecular weight of...
The vapour pressure of pure liquids A and B are 450 and 700 mm Hg respectively, at 350 K. Find out the composition of the liquid mixture if total vapour pressure is 600 mm Hg. Also find the composition of the vapour phase.
As we know that: $P_{A}^{\circ}=450 \mathrm{~mm} \text { of } \mathrm{Hg}$ $P_{B}^{\circ}=700 \mathrm{~mm}$ of $\mathrm{Hg}$ $P_{\text {total }}=600 \mathrm{~mm} \text { of } \mathrm{Hg}$ As...
Calculate Henry’s law constant when the solubility of H2S (a toxic gas with rotten egg like smell) in water at STP is 0.195 m
It is assumed that the solubility of $\mathrm{H}_{2} \mathrm{~S}$ in water at STP is $0.195 \mathrm{~m}$, which means that $0.195 \mathrm{~mol}$ of $\mathrm{H}_{2} \mathrm{~S}$ dissolves in $1000...
If 1.202 g mL^{-1}mL−1 is the density of 20% aqueous KI, determine the following:
(a) Molality of KI (b) Molarity of KI (c) Mole fraction of KI (a) Molar mass of $\mathrm{Kl}=39+127=166 \mathrm{~g} \mathrm{~mol}^{-1}$ $20 \%$ aqueous solution of KI shows that $20 \mathrm{~g}$ of...
To make 2.5 kg of 0.25 molar aqueous solution, determine the mass of urea (NH2CONH2) that is required.
Urea’s molar mass (NH2CONH2) = $2(1 \times 14+2 \times 1)+1 \times 12+1 \times 16=60 \mathrm{~g} \mathrm{~mol}^{-1}$ 0.25 urea in a molar aqueous solution denotes: $1000 \mathrm{~g}$ of water...
Determine the molarity of each of the solutions given below:
(a) 30 g of Co(NO)3. 6H2O in 4.3 L of solution (b) 30 mL of 0.5 M H2SO4 diluted to 500 mL. We are aware of this., Molarity $=\frac{\text { Moles of Solute }}{\text { Volume of solution in litre...
If benzene in solution containing 30% by mass in carbon tetrachloride, calculate the mole fraction of benzene.
Assume benzene has a mass of $30 \mathrm{~g}$ in a solution with a total mass of $100 \mathrm{~g}$. Mass of $\mathrm{CCl}_{4}=(100-30) \mathrm{g}$ $=70 \mathrm{~g}$ benzene’s molar mass...
If 22 g of benzene is dissolved in 122 g of carbon tetrachloride, determine the mass percentage of carbon tetrachloride (CCl4) and benzene (C6H6).
Benzene's mass percent $\left(\mathrm{C}_{6} \mathrm{H}_{6}\right)=\frac{\text { Mass of } C_{6} H_{6}}{\text { Total mass of the solution }} v \times 100$ $=\frac{\text { Mass of } C_{6}...
What will be the correct order for the wavelengths of absorption in the visible region for the following:
![Rendered by QuickLaTeX.com \[{{[\mathbf{Ni}{{(\mathbf{N}{{\mathbf{O}}_{\mathbf{2}}})}_{\mathbf{6}}}]}^{\mathbf{4}}},\text{ }{{[\mathbf{Ni}{{(\mathbf{N}{{\mathbf{H}}_{\mathbf{3}}})}_{\mathbf{6}}}]}^{\mathbf{2}+}},\text{ }{{[\mathbf{Ni}{{({{\mathbf{H}}_{\mathbf{2}}}\mathbf{O})}_{\mathbf{6}}}]}^{\mathbf{2}+}}?\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c719fbf2dc2de970f85896e939d068d0_l3.png)
Because all of the complexes have the same metal ion, the energy absorption is determined by the ligands' CFSE values. The ligands' CFSE values are in the order of H2O < NH3 < NO2- according...
Amongst the following, the most stable complex is ![Rendered by QuickLaTeX.com \[\left( i \right)\text{ }{{[Fe{{({{H}_{2}}O)}_{6}}]}^{3+}}\left( ii \right)\text{ }{{[Fe{{(N{{H}_{3}})}_{6}}]}^{3+}}~\left( iii \right)\text{ }{{[Fe{{({{C}_{2}}{{O}_{4}})}_{3}}]}^{3}}~~~\left( iv \right)\text{ }{{[FeC{{l}_{6}}]}^{3}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c9055c71fe68a5c25b0ec883d67368e1_l3.png)
Fe has an oxidation state of +3 in all circumstances. The chelating ligand (C2O4)3 is a bidentate chelating ligand that produces chelating rings. As a result, the most stable complex is ( iii ).
What is the oxidation number of cobalt in K[Co(CO)4]?
Amongst the following ions which one has the highest magnetic moment value? ![Rendered by QuickLaTeX.com \[\left( i \right)\text{ }{{[Cr{{({{H}_{2}}O)}_{6}}]}^{3+}}\left( ii \right)\text{ }{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\left( iii \right)\text{ }{{[Zn{{({{H}_{2}}O)}_{6}}]}^{2+}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-a172782a885937508308ebe32dd3d3ec_l3.png)
How many ions are produced from the complex Co(NH3)6Cl2 in solution?
(i) 6 (ii) 4 (iii) 3 (iv) 2
(iii) The answer is 3. The given complex [ Co( NH3)6 ]Cl2 ionizes to give three ions, viz one [ Co( NH3)6] + and two Cl – ions.
What can be inferred from the magnetic moment values of the following complex species?
Example Magnetic Moment (BM)
K4[Mn(CN)6) 2.2
[Fe(H2O)6]2+ 5.3
K2[MnCl4] 5.9
Magnetic moment is calculated by the formula: ν=[n(n+2)]1/2 For value n = 1, ν=[1(1+2)]1/2= (3)1/2 = 1.732 For value n = 2, ν=[2(2+2)]1/2= (8)1/2 = 2.83 For value n = 3, ν=[3(3+2)]1/2 =...
Discuss briefly giving an example in each case the role of coordination compounds in:
(i) analytical chemistry and (ii) extraction/metallurgy of metals.
(i) Role in analytical chemistry:Determination of hardness of the water. (ii) Complexes are generated during the extraction of metals from ores, and they play a role in metallurgy and...
Discuss briefly giving an example in each case the role of coordination compounds in:
(i) biological systems (ii) medicinal chemistry
(i) Role in biological systems: There are various essential coordination compounds in the bodies of animals, such as hemoglobin, which is an iron coordination compound. In plants, chlorophyll...
Comment on the statement that elements of the first transition series possess many properties different from those of heavier transition elements.
In many ways, the properties of elements of heavier transition elements differ from those of the first transition series. (a) The elements of the first transition series form low-spin or high-spin...
What is meant by the chelate effect? Give an example.
The metal-ligand bond becomes more stable when a polydentate or bidentate ligand attaches to a metal ion in such a way that it takes on the structure of a ring. Chelate rings are the name for these...
Explain the violet color of the complex ![Rendered by QuickLaTeX.com \[{{[\mathbf{Ti}{{({{\mathbf{H}}_{\mathbf{2}}}\mathbf{O})}_{\mathbf{6}}}]}^{\mathbf{3}+}}~\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-a69eb6951f610e97c75cb1f3a64bd20f_l3.png)
on the basis of crystal field theory.
The degree/level of association among the species involved in a state of equilibrium determines the stability of a coordination molecule in a solution. The formation constant or stability constant...
Write down the number of 3d electrons in each of the following ions: Ti2+, V2+, Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+ and Cu2+. Indicate how would you expect the five 3d orbitals to be occupied for these hydrated ions (octahedral).
Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points: (i) ionisation enthalpies and (ii) atomic sizes
In each of the three transition series, generally the first ionisation enthalpy increases from left to right. However, there are some exceptions. The first ionisation enthalpies of the third...
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration, and coordination number. Also, give stereochemistry and magnetic moment of the complex: ![Rendered by QuickLaTeX.com \[\left( i \right)\text{ }[CrC{{l}_{3}}{{\left( py \right)}_{3}}]\left( ii \right)\text{ }Cs[FeC{{l}_{4}}]\left( iii \right)\text{ }{{K}_{4}}[Mn{{\left( CN \right)}_{6}}]\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-94b27372566420147b9890b0383935a6_l3.png)
Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points: (i) electronic configurations (ii) oxidation states
(i) In the 1st, 2nd and 3rd transition series, the 3d, 4d, and 5d orbitals are filled with electrons respectively. We know that elements in the same group generally have similar electronic...
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also, give stereochemistry and magnetic moment of the complex:
(i) K[Cr(H2O)2(C2O4)2].3H2O (ii) [Co(NH3)5Cl]Cl2
n = 0.Thus, Magnetic moment = 0
Give the oxidation state, d orbital occupation and coordination number of
the central metal ion in the following complexes:
(i) (NH4)2[CoF4] (ii) [Mn(H2O)6]SO4
Give the oxidation state, d orbital occupation and coordination number of
the central metal ion in the following complexes:
(i) K3[Co(C2O4)3] (iii) (NH4)2[CoF4]
Discuss the nature of bonding in metal carbonyls.
The metal-carbon bond in metal carbonyls has both the and bond characteristics. The carbonyl carbon donates a lone pair of electrons to the metal's vacant orbital, forming a connection. The filled d...
