(i) HF (ii) HCl (iii) HBr (iv) HI Correct Answer: (i) HF Explanation: HF is the halogen acids that must have the highest bond dissociation enthalpy.
Bond dissociation enthalpy of E—H (E = element) bonds are given below. Which of the compounds will act as the strongest reducing agent?
Compound∆diss (E—H)/kJ mol–1 NH3389 PH3322 AsH3297 SbH3255 (i) NH3 (ii) PH3 (iii) AsH3 (iv) SbH3 Correct Answer: (iv) SbH3 Explanation: SbH3 is the compound that will act as the strongest...
On heating with concentrated NaOH solution in an inert atmosphere of CO2, white phosphorus gives a gas. Which of the following statement is incorrect about the gas?
(i) It is highly poisonous and has smelled like rotten fish. (ii) It’s a solution in water decomposes in the presence of light. (iii) It is more basic than NH3 (iv) It is less basic than NH3 ...
Which of the following acids forms three series of salts?
(i) H3PO2 (ii) H3BO3 (iii) H3PO4 (iv) H3PO3 Correct Answer: (iii) H3PO4 Explanation: H3PO4 is the acid that form three series of salts.
Strong reducing behaviour of H3PO2 is due to
(i) The low oxidation state of phosphorus (ii) Presence of two –OH groups and one P–H bond (iii) Presence of one –OH group and two P–H bonds (iv) High electron gain enthalpy of phosphorus ...
On heating lead, nitrate forms oxides of nitrogen and lead. The oxides formed are ______.
(i) N2O, PbO (ii) NO2, PbO (iii) NO, PbO (iv) NO, PbO2 Correct Answer: (ii) NO2, PbO Explanation: Reaction Involved - 2Pb(NO3)2 → 2PbO + 4NO2 + O2
Which of the following elements does not show allotropy?
(i) Nitrogen (ii) Bismuth (iii) Antimony (iv) Arsenic Correct Answer: (i) Nitrogen Explanation: The element that do not show allotropy is Nitrogen.
Maximum covalency of nitrogen is ______________.
(i) 3 (ii) 5 (iii) 4 (iv) 6 Correct Answer: (iii) 4 Explanation: Maximum covalency of nitrogen is 4.
Which of the following statements is wrong?
(i) Single N–N bond is stronger than the single P–P bond. (ii) PH3 can act as a ligand in the formation of a coordination compound with transition elements. (iii) NO2 is paramagnetic. (iv) Covalency...
If A, B and C are angles of a triangle, then the determinant is equal to (A) 0 (B) -1 (C) 1 (D) None of these
Answer: \[Option\text{ }\left( A \right)\text{ }0\]
A brown ring is formed in the ring test for NO3– ion. It is due to the formation of
(i) [Fe(H2O)5 (NO)]2+ (ii) FeSO4.NO2 (iii) [Fe(H2O)4 (NO)]2+ (iv) FeSO4.HNO3 Correct Answer: (i) [Fe(H2O)5 (NO)]2+ Explanation: A brown ring is formed in the ring test for NO3– ion. It is...
Elements of group-15 form compounds in +5 oxidation state. However, bismuth forms only one well-characterised compound in +5 oxidation state. The compound is
(i) Bi2O5 (ii) BiF5 (iii) BiCl5 (iv) Bi2S5 Correct Answer: (ii) BiF5 Explanation: Due to the inert pair effect bismuth only exhibit +3 oxidation state and form trihalides. But due to the size...
On heating ammonium dichromate and barium azide separately we get
(i) N2 in both cases (ii) N2 with ammonium dichromate and NO with barium azide (iii) N2O with ammonium dichromate and N2 with barium azide (iv) N2O with ammonium dichromate and NO2 with barium azide...
Find the component statements of the following compound statements.
(i) √7 is a rational number or an irrational number.
(ii) 0 is less than every positive integer and every negative integer.
(i) A compound statement is made up of two or more statements (Components). As a result, the components of the given statement 7are a rational or irrational number, respectively. p: √7is a rational...
The number of distinct real roots of = 0 in the interval -π/4 ≤ x ≤ π/4 is (A) 0 (B) 2 (C) 1 (D) 3
Answer: \[Option\text{ }\left( C \right)\text{ }1\]
The determinant equals (A) abc (b–c) (c – a) (a – b) (B) (b–c) (c – a) (a – b) (C) (a + b + c) (b – c) (c – a) (a – b) (D) None of these
Answer: Option (D) \[None\text{ }of\text{ }these\]
Find the component statements of the following compound statements.
(i) Number 7 is prime and odd.
(ii) Chennai is in India and is the capital of Tamil Nadu.
(i) A compound statement is made up of two or more statements (Components). As a result, the elements of the provided statement "Number 7 is prime and odd" are as follows: p: The number 7 is prime....
The area of a triangle with vertices (–3, 0), (3, 0) and (0, k) is 9 sq. units. The value of k will be (A) 9 (B) 3 (C) – 9 (D) 6
Option \[\left( B \right)\text{ }3\] According to the question, the area of a triangle with vertices \[({{x}_{1}},\text{ }{{y}_{1}}),\text{ }({{x}_{2}},\text{ }{{y}_{2}})\text{ }and\text{...
The value of determinant (A) a3 + b3 + c3 (B) 3 bc (C) a3 + b3 + c3 – 3abc (D) none of these
ANSWER: Option \[\left( C \right)~{{a}^{3}}~+~{{b}^{3}}~+~{{c}^{3}}~\text{ }3abc\] Given,
If then, value of x is (A) 3 (B) ± 3 (C) ± 6 (D) 6
ANSWER: Option \[~\left( C \right)\text{ }\pm \text{ }6\] On equating the determinants, we get \[2{{x}^{2}}-\text{ }40\text{ }=\text{ }18\text{ }+\text{ }14\] \[2{{x}^{2}}~=\text{ }72\]...
If x + y + z = 0, prove that
Answer: According to the question, the determinant can be solved as
Prove that is divisible by a + b + c and find the quotient.
Answer: According to the question, the determinant can be solved as
If a + b + c ¹ 0 and then prove that a = b = c.
Answer: According to the question, the determinant can be solved as
Given find BA and use this to solve the system of equations y + 2z = 7, x – y = 3, 2x + 3y + 4z = 17.
Answer: According to the question, the determinant can be solved as
Using matrix method, solve the system of equations 3x + 2y – 2z = 3, x + 2y + 3z = 6, 2x – y + z = 2.
Answer: Given equations are: \[3x~+\text{ }2y-\text{ }2z~=\text{ }3\] \[x~+\text{ }2y~+\text{ }3z~=\text{ }6\]and \[2x~-y~+~z~=\text{ }2\] Or, \[AX\text{ }=\text{ }B\]
Find the area enclosed by the curve y = –x^2 and the straight-line x + y + 2 = 0.
The curve y = –x2 or x2 = –y and the line x + y + 2 = 0 Solving the two equation, we get \[x\text{ }-\text{ }{{x}^{2}}~+\text{ }2\text{ }=\text{ }0\] \[{{x}^{2}}~\text{ }-x\text{ }\text{ }-2\text{...
If A = , find A-1. Using A–1, solve the system of linear equations x – 2y = 10 , 2x – y – z = 8 , –2y + z = 7.
Answer: According to the question, the determinant can be solved as
Find A–1 if and show that A-1 = (A2 – 3I)/ 2.
Answer: According to the question, the determinant can be solved as
Show that the DABC is an isosceles triangle if the determinant
Answer: According to the question, the determinant can be solved as
Show that the points (a + 5, a – 4), (a – 2, a + 3) and (a, a) do not lie on a straight line for any value of a.
Given focuses are \[\left( a\text{ }+\text{ }5,\text{ }a\text{ }\text{ }4 \right),\text{ }\left( a\text{ }\text{ }2,\text{ }a\text{ }+\text{ }3 \right)\]and \[\left( a,\text{ }a \right).\]...
If a1, a2, a3, …, ar are in G.P., then prove that the determinant is independent of r.
Answer: According to the question, the determinant can be solved as So, it is independent of r.
If , then find values of x.
Answer: According to the question, the determinant can be solved as
Find the value of q satisfying
Answer: According to the question, the determinant can be solved as
Using integration, find the area of the region bounded by the line 2y = 5x + 7, x-axis and the lines x = 2 and x = 8.
Given, \[2y~=\text{ }5x~+\text{ }7,~x-axis,~x~=\text{ }2\text{ }and~x~=\text{ }8\] graphg for: \[2y~=\text{ }5x~+\text{ }7\text{ }\Rightarrow \text{ }y\text{ }=\text{ }\left( 5x\text{ }+\text{ }7...
If the co-ordinates of the vertices of an equilateral triangle with sides of length ‘a’ are (x1, y1),(x2, y2), (x3, y3), then
Answer: According to the question, the determinant can be solved as
If A + B + C = 0, then prove that
Answer: According to the question, the determinant can be solved as
Find the area of region bounded by the line x = 2 and the parabola y^2 = 8x
The equation of line x = 2 and parabola y2 = 8x Putting value of x in the other equation, we have \[\begin{array}{*{35}{l}} {{y}^{2}}~=\text{ }8\left( 2 \right) \\ {{y}^{2}}~=\text{ }16 \\...
Why first ionization enthalpy of Cr is lower than that of Zn?
Solution: The electronic setup of chromium and zinc are separately: \[\begin{array}{*{35}{l}} Cr\text{ }\left( 24 \right)\text{ }=\text{ }\left[ Ar \right]\text{ }3d54s2 \\ ~Zn\text{ }\left(...
Prove that:
Answer: According to the question, the determinant can be solved as
Prove that:
Answer: According to the question, the determinant can be solved as
Prove that:
Answer: According to the question, the determinant can be solved as
Evaluate the following determinant:
Answer: According to the question, the determinant can be solved as Now, expanding along\[R1\], we have \[=\text{ }\left( a\text{ }+\text{ }b\text{ }+\text{ }c \right)\text{ }[1\text{ }x\text{...
Evaluate the following determinant:
Answer: According to the question, the determinant can be solved as
Assertion: Hydrometallurgy involves dissolving the ore in a suitable reagent followed by precipitation by a more electropositive metal. Reason: Copper is extracted by hydrometallurgy.
(i) Both assertion and reason are true and the reason is the correct explanation of assertion. (ii) Both assertion and reason are true but the reason is not the correct explanation of assertion....
Assertion: Zone refining method is very useful for producing semiconductors. Reason: Semiconductors are of high purity.
(i) Both assertion and reason are true and the reason is the correct explanation of assertion. (ii) Both assertion and reason are true but the reason is not the correct explanation of assertion....
Evaluate the following determinant:
Answer: [Expanding along first column] \[=\text{ }\left( x\text{ }+\text{ }y\text{ }+\text{ }z \right)\text{ }.\text{ }1\left[ 3y\left( 3z\text{ }+\text{ }x \right)\text{ }+\text{ }\left(3z...
Assertion: Sulphide ores are concentrated by Froth Flotation method. Reason: Cresols stabilise the froth in the Froth Flotation Method.
(i) Both assertion and reason are true and the reason is the correct explanation of assertion. (ii) Both assertion and reason are true but the reason is not the correct explanation of assertion....
Assertion: Zirconium can be purified by Van Arkel method. Reason: ZrI4 is volatile and decomposes at 1800K.
(i) Both assertion and reason are true and the reason is the correct explanation of assertion. (ii) Both assertion and reason are true but the reason is not the correct explanation of assertion....
Assertion: Nickel can be purified by the Mond process. Reason: Ni (CO)4 is a volatile compound which decomposes at 460K to give pure Ni.
(i) Both assertion and reason are true and the reason is the correct explanation of assertion. (ii) Both assertion and reason are true but the reason is not the correct explanation of assertion....
Evaluate the following determinant:
Answer: According to the question, the determinant can be solved as
Evaluate the following determinant:
Answer: According to the question, the determinant can be solved as
Evaluate the following determinant:
Answer: \[=\text{ }({{x}^{2}}-\text{ }2x\text{ }+\text{ }2)\text{ }.\text{ }\left( x\text{ }+\text{ }1 \right)\text{ }-\text{ }\left( (x)\text{ }-\text{ }1 \right)\text{ }.\text{ }0\] \[=\text{...
solve the following:
Solution:
solve the following:
Solution:
Match the items of Column I with items of Column II.
Column I Column II (A) Pendulum (1) Chrome steel (B) Malachite (2) Nickel steel (C) Calamine (3) Na3AlF6 (D) Cryolite (4) CuCO3.Cu(OH)2 (5) ZnCO3 Column I Column II (A) Pendulum (2)...
Match the items of Column I with the items of Column II
Column I Column II (A) Coloured band (1) Zone refining (B) Impure metal to volatile complex (2) Fractional distillation (C) Purification of Ge and Si (3) Mond Process (D) Purification of mercury (4)...
Match items of Column I with the items of Column II
Column I Column II (A) Cyanide process (1) Ultrapure Ge (B) Froth Floatation Process (2) Dressing of ZnS (C) Electrolytic reduction (3) Extraction of Al (D) Zone refining (4) Extraction of Au ...
Match the items of Column I with the items of Column II
Column I Column II (A) Sapphire (1) Al2O3 (B) Sphalerite (2) NaCN (C) Depressant (3) Co (D) Corundum (4) ZnS (5) Fe2O3 Column I Column II (A) Sapphire (3) Co (B) Sphalerite (4) ZnS (C)...
Match the items of Column I with items of Column II
Column I Column II (A) Blistered Cu (1) Aluminium (B) Blast furnace (2) 2Cu2O + Cu2S → 6Cu + SO2 (C) Reverberatory furnace (3) Iron (D) Hall-Heroult process (4) FeO + SiO2 → FeSiO3 (5) 2Cu2S...
Find which of the functions if is continuous or discontinuous at the indicated points:
at \[\mathbf{x}\text{ }=\text{ }\mathbf{4}\] And hence, f(x) is discontinuous at \[x\text{ }=\text{ }4.\]
Find which of the functions if is continuous or discontinuous at the indicated points:
at \[\mathbf{x}\text{ }=\text{ }\mathbf{2}\] And hence, f(x) is continuous at \[x\text{ }=\text{ }2.\]
Find which of the functions if is continuous or discontinuous at the indicated points:
at \[\mathbf{x}\text{ }=\text{ }\mathbf{2}\] And hence, f(x) is discontinuous at \[x\text{ }=\text{ }2.\]
Write the chemical reactions involved in the extraction of gold by the cyanide process. Also, give the role of zinc in the extraction.
Reactions Involved: 4Au(s) + 8CN-(aq) + 2H2O(aq) + O2(g) → 4[Au(CN)2]-(aq) + 4OH-(aq) 2[Au(CN)2]-(aq) + Zn(s) → 2Au(s) + [Zn(CN)4]2- (aq) Role of Zinc in this reaction - Reducing...
Give two requirements for vapour phase refining.
Requirements for vapour phase refining: 1. Metal must react easily with a reagent to produce volatile complex. 2. The complex which is volatile should be easily decomposed and recovered.
Write down the reactions taking place in Blast furnace-related to the metallurgy of iron in the temperature range 500-800 K.
Reactions Involved: 1. 3Fe2O3 + CO → 2Fe3O4 + CO2 2. Fe3O4 + CO → 3FeO + 2CO2 3. Fe2O3 + CO → 2FeO + CO2
How are metals used as semiconductors refined? What is the principle of the method used?
1. Semiconductors are purified by the method of zone refining. 2. The refining of the surface depends on the principle that the impurities are more soluble in molten metal than solid metal. 3. The...
What is the role of flux in metallurgical processes?
Role of flux in metallurgical processes: 1. To remove the gangue, certain substances are mixed with it. These are called fluxes. 2. Flux can be basic or acidic. 3. Flux which is acidic removes basic...
What should be the considerations during the extraction of metals by electrochemical method?
Considerations during the extraction of metals by electrochemical method: 1. The reactivity of the formed metal. 2. Electrodes must be made of suitable materials 3. To form a molten mass, flux is...
Why is an external emf of more than 2.2V required for the extraction of Cl2 from brine?
Reaction involved in the extraction of Cl2 from brine: 2Cl- (aq) + 2H2O(l) → 2OH- (aq) + H2(g) + Cl2(g) Calculation: ΔGᶱ = -nFE° ΔGᶱ = +422kJmol-1. E° = 422x103 / 2×96500 E° = - 2.2V For the...
At temperatures above 1073K coke can be used to reduce FeO to Fe. How can you justify this reduction with the Ellingham diagram?
The ∆G formation of FeO is a bit negative than the ∆G formation of carbon monoxide from carbon. Summation of both ∆G will be negative at about 1073K. Above 1073K the FeO production line exceeds the...
Wrought iron is the purest form of iron. Write a reaction used for the preparation of wrought iron from cast iron. How can the impurities of sulphur, silicon and phosphorus be removed from cast iron?
Limestone can be combined with flux to remove impurities of sulfur, silicon and phosphorus. They form an easily removable slag. The metal is removed from the slag by passing through the rollers....
How is copper extracted from low-grade copper ores?
Copper is extracted by hydrometallurgy method from the copper ores which are low graded. The Cu is treated with cast iron or H2 and is leached out by the acid. Reaction Involved: Cu2 (aq) + H2(g) →...
Write two basic requirements for refining of metal by Mond process and by Van Arkel Method.
1. The metal must react easily with a reagent to produce complex. 2. The complex which is volatile must be easily decomposed and do not give off any additional products and recovery must also be...
Although carbon and hydrogen are better-reducing agents they are not used to reduce metallic oxides at high temperatures. Why?
Carbon and hydrogen will not reduce oxides into metals but will instead build hydrides and carbides. Thus, they are not used as the reducing agents.
How do we separate two sulphide ores by Froth Floatation Method? Explain with an example.
The two sulphide ores competing with coming into the froth can be separated by adjusting the oil to the amount of water or by adding depressants such as NaCN. If we have two sulphide metals, ZnS and...
The purest form of iron is prepared by oxidising impurities from cast iron in a reverberatory furnace. Which iron ore is used to line the furnace? Explain by giving a reaction.
The iron ore which is used to line the reverberatory furnace is Haematite ore (Fe2O3). Example: In the following reaction, Haematite ore forms iron and CO. Fe2O3 + 3 C → 2 Fe + 3CO
The mixture of compounds A and B is passed through a column of Al2O3 by using alcohol as eluant. Compound A is eluted in preference to compound B. Which of the compounds A or B, is more readily adsorbed on the column?
Compound A does not absorb well in the column and moves down the column with alcohol i.e. eluant, while compound B is well absorbed in the column and cannot go down the column.
Why is sulphide ore of copper heated in a furnace after mixing with silica?
The copper sulphide ore is burned in the furnace after mixing with silica because the metal impurities present in it can form slag and silica and will be easily removed. Copper is produced as matte...
Why are sulphide ores converted to oxide before reduction?
The Sulphide ores cannot be easily reduced so they are usually converted to oxides because the oxides can be easily reduced.
Which method is used for refining Zr and Ti? Explain with the equation.
The Van Arkel method is used to filter Zr and Ti. Van Arkel's process involves heating the impure metal with Iodine. It builds a complex structure where it decomposes at high temperatures, restores...
Brine is electrolysed by using inert electrodes. The reaction at anode is ________.
(i) Cl–(aq.) →1/2Cl2 (g) + e– ; E°cell = 1.36V (ii) 2H2O(l) → O2(g0 + 4H+ + 4e- ; E°cell = 1.23V (iii) Na+(aq) + e- → Na(s) ; E°cell = 2.71V (iv) H+(aq) + e- → 1/2H2(g) ; E°cell = 0.00V ...
In the metallurgy of aluminium ________________.
(i) Al3+ is oxidised to Al (s). (ii) graphite anode is oxidised to carbon monoxide and carbon dioxide. (iii) the oxidation state of oxygen changes in the reaction at the anode. (iv) the oxidation...
Electrolytic refining is used to purify which of the following metals?
(i) Cu and Zn (ii) Ge and Si (iii) Zr and Ti (iv) Zn and Hg Correct Answer: (i) Cu and Zn Explanation: Electrolytic refining is used to purify Copper and Zinc metals.
Extraction of gold and silver involves leaching the metal with CN–ion. The metal is recovered by ________________.
(i) displacement of metal by some other metal from the complex ion. (ii) roasting of the metal complex. (iii) calcination followed by roasting. (iv) thermal decomposition of the metal complex....
Choose the correct option of temperature at which carbon reduces FeO to iron and produces CO.
(i) Below temperature at point A. (ii) Approximately at the temperature corresponding to point A. (iii) Above temperature at point A but below a temperature at point D. (iv) Above temperature at...
Below point ‘A’ FeO can ______________.
(i) be reduced by carbon monoxide only. (ii) be reduced by both carbon monoxide and carbon. (iii) be reduced by carbon only. (iv) not be reduced by both carbon and carbon monoxide. Correct...
For the reduction of FeO at the temperature corresponding to point D, which of the following statements is correct?
(i) ∆G value for the overall reduction reaction with carbon monoxide is zero. (ii) ∆G value for the overall reduction reaction with a mixture of 1 mol carbon and 1 mol oxygen is positive. (iii) ∆G...
At the temperature corresponding to which of the points in the Figure, FeO will be reduced to Fe by coupling the reaction 2FeO → 2Fe + O2 with all of the following reactions?
(a) C + O2 → CO2 (b) 2C + O2 → 2CO (c) 2CO + O2 → 2 CO2 (i) Point A (ii) Point B (iii) Point D (iv) Point E Correct Answers: (ii) Point B (iv) Point E Explanation: At the temperature...
Which of the following option are correct?
(i) Cast iron is obtained by remelting pig iron with scrap iron and coke using hot air blast. (ii) In the extraction of silver, silver is extracted as cationic complex. (iii) Nickel is purified by...
In the extraction of aluminium by Hall-Heroult process, purified Al2O3 is mixed with CaF2 to
(i) lower the melting point of Al2O3 (ii) increase the conductivity of molten mixture. (iii) reduce Al3+ into Al(s). (iv) acts as a catalyst Correct Answers: (i) lower the melting point of...
Which of the following statements is correct about the role of substances added in the froth floatation process?
(i) Collectors enhance the non-wettability of the mineral particles. (ii) Collectors enhance the wettability of gangue particles. (iii) By using depressants in the process two sulphide ores can be...
In the Froth Floatation process, zinc sulphide and lead sulphide can be separated by ______________.
(i) using collectors. (ii) adjusting the proportion of oil to water. (iii) using depressant. (iv) using froth stabilisers. Correct Answers: (ii) adjusting the proportion of oil to water....
Common impurities present in bauxite are ____________.
(i) CuO (ii) ZnO (iii) Fe2O3 (iv) SiO2 Correct Answers: (iii) Fe2O3 (iv) SiO2 Explanation: Common impurities present in bauxite are Fe2O3 and SiO2
Which of the following ores are concentrated by froth flotation?
(i) Haematite (ii) Galena (iii) Copper pyrites (iv) Magnetite Correct Answers: (ii) Galena (iii) Copper pyrites Explanation: Galena and Copper pyrites are the ores which can be concentrated...
Which of the following reactions occur during calcination?
(i) CaCO3→CaO+CO2 (ii) 4FeS2+11O2→2Fe2O3+8SO2 (iii) 2Al(OH)3→Al2O3+3H2O (iv) Cu2S+2CuO→4Cu+SO2 Correct Answers: (i) CaCO3→CaO+CO2 (iii) 2Al(OH)3→Al2O3+3H2O Explanation:...
For the metallurgical process of which of the ores calcined ore can be reduced by carbon?
(i) haematite (ii) calamine (iii) iron pyrites (iv) sphalerite Correct Answers: (i) haematite (ii) calamine Explanation: Haematite and Calamine are the calcined ores which gets reduced by...
The main reactions occurring in the blast furnace during the extraction of iron from haematite are ________.
(i) Fe2O3 + 3CO → 2Fe + 3CO2 (ii) FeO + SiO2 → FeSiO3 (iii) Fe2O3 + 3C → 2Fe + 3CO (iv) CaO + SiO2 → CaSiO3 Correct Answers: (i) Fe2O3 + 3CO → 2Fe + 3CO2 (iv) CaO + SiO2 → CaSiO3 Explanation:...
In which of the following method of purification, metal is converted to its volatile compound which is decomposed to give pure metal?
(i) heating with a stream of carbon monoxide. (ii) heating with iodine. (iii) liquation. (iv) distillation. Correct Answers: (i) heating with a stream of carbon monoxide (ii) heating with...
Which of the following statements are correct?
(i) A depressant prevents a certain type of particle to come to the froth. (ii) Copper matte contains Cu2S and ZnS. (iii) The solidified copper obtained from the reverberatory furnace has blistered...
In the extraction of chlorine from brine _____________.
(i) ΔG° for the overall reaction is negative. (ii) ΔG° for the overall reaction is positive. (iii) E° for the overall reaction has a negative value. (iv) E° for the overall reaction has a positive...
Find which of the functions if is continuous or discontinuous at the indicated points:
at \[\mathbf{x}\text{ }=\text{ }\mathbf{0}\] NOW, HENCE, given function f(x) is discontinuous at \[x\text{ }=\text{ }0.\]
Examine the continuity of the function f (x) = x3 + 2×2 – 1 at x = 1
since, \[y\text{ }=\text{ }f\left( x \right)\] will be continuous at \[x\text{ }=\text{ }a\] if, hence, f(x) is continuous at $$ \[x\text{ }=\text{ }1.\]
In the extraction of chlorine by electrolysis of brine ____________.
(i) oxidation of Cl– ion to chlorine gas occurs. (ii) reduction of Cl– ion to chlorine gas occurs. (iii) For the overall reaction, ∆Gᶱ has a negative value. (iv) a displacement reaction takes place....
When copper ore is mixed with silica, in a reverberatory furnace copper matte is produced. The copper matte contains ____________.
(i) sulphides of copper (II) and iron (II) (ii) sulphides of copper (II) and iron (III) (iii) sulphides of copper (I) and iron (II) (iv) sulphides of copper (I) and iron (III) Correct...
Which of the following reactions is an example of autoreduction?
(i) Fe3O4 + 4CO → 3Fe + 4CO2 (ii) Cu2O + C → 2Cu + CO (iii) Cu2+ (aq) + Fe (s) → Cu (s) + Fe2+ (aq) (iv) Cu2O + 1/2Cu2S → 3Cu + 1/2SO2 Correct Answer: (iv) Cu2O + 1/2Cu2S → 3Cu + 1/2SO2...
A number of elements are available in earth’s crust but most abundant elements are ____________.
(i) Al and Fe (ii) Al and Cu (iii) Fe and Cu (iv) Cu and Ag Correct Answer:(i) Al and Fe Explanation: The most abundant elements in the earth's crust are Aluminium (8.1 %) and Iron...
In the extraction of copper from its sulphide ore, the metal is formed by the reduction of Cu2O with
(i) FeS (ii) CO (iii) Cu2S (iv) SO2 Correct Answer: (iii) Cu2S Explanation: Reaction Involved - 2Cu2S+3O2 →2Cu2O+2SO2 2 Cu2O+ Cu2S →6Cu+ SO2
Zone refining is based on the principle that ___________
(i) impurities of low boiling metals can be separated by distillation. (ii) impurities are more soluble in molten metal than in solid metal. (iii) different components of a mixture are differently...
Find the term independent of 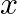 in the expansion of
in the expansion of 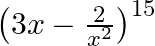
Given function is $\left(3 x-\frac{2}{x^{2}}\right)^{15}$ We know from the standard formula of $T_{r+1}$ we get, $\mathrm{T}_{r+1}={ }^{15} C_{r}(3 x)^{15-r}\left(\frac{-2}{x^{2}}\right)^{r}={...
Why is Fe(OH)3 colloid positively charged, when prepared by adding FeCl3 to hot water?
When FeCl3 is dissolved in hot water, hydrated ferric oxide forms. This highly hydrated oxide hydrate adsorbs Fe3+ ions results in a positively charged colloid.
Why do physisorption and chemisorption behave differently with rising in temperature?
1. In physisorption, with increasing temperature, the bond between adsorbent and adsorbate weakens and the amount of adsorbate decreases. 2. In chemisorption, the amount of activation energy needed...
What happens when dialysis is prolonged?
If dialysis continues for a long time, the electrolyte deposits present in the blood are also completely removed and blood coagulation is established.
Why does the white precipitate of silver halide become coloured in the presence of dye eosin?
The silver halide surface acts as a good adsorbent. It can be able to adsorb the pigment present in the eosin dye. So, the silver halide seems to be colored.
What is the role of activated charcoal in a gas mask used in coal mines?
The activated charcoal works as a good adsorbent because it is porous. Because of this, in the coal mines, it is used to provide fresh air for the breathing purpose via a gas mask.
An audio signal is modulated by a carrier wave of 20MHz such that the bandwidth required for modulation is 3kHz. Could this wave be demodulated by a diode detector which has the values of R and C as
(i) R = 1 kΩ, C = 0.01µF (ii) R = 10 kΩ, C = 0.01µF (iii) R = 10 kΩ, C = 0.1µF Answer: Carrier wave frequency, fc – 20 MHz = 20 × 106 Hz Bandwidth = 2fm = 3 × 103 Hz fm = 1.5 × 103 Hz 1/fc = 0.5 ×...
(i) Draw the plot of amplitude versus ‘ω’ for an amplitude modulated wave whose carrier wave (ωc ) is carrying two modulating signals, ω1 and ω2 (ω2 > ω1). [Hint: Follow derivation from Eq 15.6 of NCERT Textbook of XII]
(ii) Is the plot symmetrical about ωc? Comment especially about plot in region ω < ωc (iii) Extrapolate and predict the problems one can expect if more waves are to be modulated (iv) Suggest...
An amplitude modulated wave is as shown in the figure. Calculate
(i) the percentage modulation (ii) peak carrier voltage and (iii) peak value of information voltage Answer; Maximum voltage is given by $ {{V}_{\max }}=\frac{100}{2}=50V $ Minimum voltage is given...
A 50 MHz sky wave takes 4.04 ms to reach a receiver via re-transmission from a satellite 600 km above earth’s surface. Assuming re-transmission time by satellite negligible, find the distance between source and receiver. If communication between the two was to be done by Line of Sight (LOS) method, what should size and placement of receiving and transmitting antenna be?
Answer: According to the question, the velocity of waves = 3 × 108 m/s The time to reach a receiver = 4.04 × 10-3 s we know that the height of satellite is: h = 600 km And the radius of earth = 6400...
(i) The intensity of a light pulse travelling along a communication
channel decreases exponentially with distance x according to the relation I = Ioe–αx, where I o is the intensity at x = 0 and α is the attenuation constant. Show that the intensity reduces by 75...
On radiating (sending out) an AM modulated signal, the total radiated power is due to energy carried by ωc, ωc – ωm & ωc + ωm. Suggest ways to minimise the cost of radiation without compromising on information
Answer: The total radiated power is due to ωc, (ωc – ωm) and (ωc + ωm) carrying energy. The carrier frequency is usually close to the sideband frequencies. In an amplitude modulated transmission,...
The maximum frequency for reflection of sky waves from a certain layer of the ionosphere is found to be f max = 9(Nmax) 1/2, where Nmax is the maximum electron density at that layer of the ionosphere. On a certain day, it is observed that signals of frequencies higher than 5MHz are not received by reflection from the F1 layer of the ionosphere while signals of frequencies higher than 8MHz are not received by reflection from the F2 layer of the ionosphere. Estimate the maximum electron densities of the F1 and F2 layers on that day.
Answer: Expression fpor the maximum frequency is: fmax = 9(Nmax)1/2 According to the question, for Layer F1, fmax = 5 MHz $ {{N}_{\max }}=\frac{F_{\max }^{2}}{9\times 9}=\frac{5\times...
If the whole earth is to be connected by LOS communication using space waves (no restriction of antenna size or tower height), what is the minimum number of antennas required? Calculate the tower height of these antennas in terms of earths radius?
Answer: We know that the distance or range of transmission tower is given by the expression: $dT=\sqrt{2{{h}_{T}}R}$ R represents the radius of the earth (approximately 6400 km). hT denotes the...
A TV transmission tower antenna is at a height of 20 m. How much service area can it cover if the receiving antenna is
(i) at ground level, (ii) at a height of 25 m? Calculate the percentage increase in area covered in case (ii) relative to case (i). Answer: We know that: $ {{R}_{e}}=6.4\times {{10}^{6}}m $ The...
Figure shows a communication system. What is the output power when the input signal is of 1.01mW? (gain in dB = 10 log10 (Po /Pi ).
Answer: It is given that there is a loss of signal 2dB per km. So total loss suffered in 5km = -2 x 5 = -10 dB. In both input and output amplifiers, the total gain of the signal is given by: =...
Why is an AM signal likely to be noisier than an FM signal upon transmission through a channel?
Answer: In an amplitude modulated wave, the carrier wave's instantaneous voltage varies. As a result, assuming noise as a part of the modulated signal, a noise signal can be added and received...
Compute the LC product of a tuned amplifier circuit required to generate a carrier wave of 1 MHz for amplitude modulation.
Answer: $ v=1MHz={{10}^{6}}Hz $ $ v=\frac{1}{2\pi \sqrt{LC}} $ $ Or,\sqrt{LC}=\frac{1}{2\pi v} $ $ LC=\frac{1}{{{(2\pi v)}^{2}}}=\frac{1}{4\times 3.14\times 3.14\times {{10}^{12}}} $ $...
The maximum amplitude of an A.M. wave is found to be 15 V while its minimum amplitude is found to be 3 V. What is the modulation index?
Answer: The modulation index is the ratio of the change in carrier wave amplitude to the original carrier wave amplitude. according to the question, the maximum amplitude of AM wave is Amax = Ac +...
Two waves A and B of frequencies 2 MHz and 3 MHz, respectively are beamed in the same direction for communication via skywave. Which one of these is likely to travel a longer distance in the ionosphere before suffering total internal reflection?
Answer: The refractive index rises as the frequency rises, implying that the angle of refraction is smaller for higher frequency waves. In other words, bending is less. As a result, after covering a...
Would sky waves be suitable for transmission of TV signals of 60 MHz frequency?
Answer; Skywaves will not be suitable for transmitting TV signals with a frequency of 60 MHz since the TV signals are higher than 60 MHz. It is necessary to use space wave transmission.
Which of the following would produce analogue signals and which would produce digital signals?
(i) A vibrating tuning fork (ii) Musical sound due to a vibrating sitar string (iii) Light pulse (iv) Output of NAND gate Answer: i) A vibrating tuning fork will produce an analog signal ii) musical...
In amplitude modulation, the modulation index m, is kept less than or equal to 1 because
(a) m > 1, will result in interference between the carrier frequency and message frequency, resulting in distortion (b) m > 1 will result in overlapping of both sidebands resulting into loss...
The frequency response curve in the figure for the filter circuit used for production of AM wave should be
(a) (i) followed by(ii) (b) (ii) followed by (i) (c) (iii) (d) (iv) Answer: The correct options are: (a) (i) followed by(ii) (b) (ii) followed by (i) (c) (iii) Explanation: The band pass filter...
A TV transmission tower has a height of 240 m. Signals broadcast from this tower will be received by LOS communication at a distance of (assume the radius of the earth to be 6.4 × 106 m)
(a) 100 km (b) 24 km (c) 55 km (d) 50 km Answer: The correct options are (b) 24 km (c) 55 km and (d) 50 km Explanation: $ {{h}_{T}}=240m $ $ R=6.4\times {{10}^{6}}m $ For, LOS communication we have:...
Audio sine waves of 3 kHz frequency are used to amplitude modulate a carrier signal of 1.5 MHz. Which of the following statements are true?
(a) The sideband frequencies are 1506 kHz and 1494 kHz (b) The bandwidth required for amplitude modulation is 6kHz (c) The bandwidth required for amplitude modulation is 3 MHz (d) The sideband...
An audio signal of 15kHz frequency cannot be transmitted over long distances without modulation because
(a) the size of the required antenna would be at least 5 km which is not convenient (b) the audio signal can not be transmitted through sky waves (c) the size of the required antenna would be at...
Identify the mathematical expression for amplitude modulated wave:
a) Ac sin [{ωc + k1vm(t)}t + φ ] (b) Ac sin {ωc t + φ + k2 vm(t)} (c) {Ac + k2 vm(t)} sin (ωc t + φ ) (d) Ac vm(t) sin (ωc t + φ ) Answer: The correct option is (c) {Ac + k2 vm(t)} sin (ωc t + φ )...
A basic communication system consists of
(A) transmitter (B) information source (C) user of information (D) channel (E) receiver Choose the correct sequence in which these are arranged in a basic communication system: (a) ABCDE (b) BADEC...
A male voice after modulation-transmission sounds like that of a female to the receiver. The problem is due to
a) poor selection of modulation index (selected 0 < m < 1) (b) poor bandwidth selection of amplifiers (c) poor selection of carrier frequency (d) loss of energy in transmission Answer: The...
I-V characteristics of four devices are shown in the figure.
Identify devices that can be used for modulation: (a) ‘i’ and ‘iii’ (b) only ‘iii’ (c) ‘ii’ and some regions of ‘iv’ (d) All the devices can be used. Answer: The correct option is (c) ‘ii’ and some...
A message signal of frequency ωm is superposed on a carrier wave of frequency ωc to get an amplitude modulated wave (AM). The frequency of the AM wave will be
(a) ωm (b) ωc (c) ωc + ωm/2 (d) ωc – ωm/2 Answer: The correct option is (b) ωc Explanation: Frequency of carrier wave = ωc Frequency of modulated wave = ωc
A speech signal of 3 kHz is used to modulate a carrier signal of frequency 1 MHz, using amplitude modulation. The frequencies of the sidebands will be
(a) 1.003 MHz and 0.997 MHz (b) 3001 kHz and 2997 kHz (c) 1003 kHz and 1000 kHz (d) 1 MHz and 0.997 MHz Answer: The correct option is (a) 1.003 MHz and 0.997 MHz Explanation: $ {{\omega...
A 1 KW signal is transmitted using a communication channel which provides attenuation at the rate of – 2dB per km. If the communication channel has a total length of 5 km, the power of the signal received is [gain in dB = 10 log P0/P1]
(a) 900 W (b) 100 W (c) 990 W (d) 1010 W Answer: The correct option is (b) 100 W Explanation: According to the question, Pi =1 kW = 1000W The rate of attenuation of the signal = -2dB/km Length of...
A 100m long antenna is mounted on a 500m tall building. The complex can become a transmission tower for waves with λ
(a) ~ 400 m (b) ~ 25 m (c) ~ 150 m (d) ~ 2400 m Answer: The correct option is (a) ~ 400 m Explanation: According to the question, the length of building ois l = 500m and Length of antenna is 100m...
Three waves A, B and C of frequencies 1600 kHz, 5 MHz and 60 MHz, respectively are to be transmitted from one place to another. Which of the following is the most appropriate mode of communication:
(a) A is transmitted via space wave while B and C are transmitted via skywave (b) A is transmitted via ground wave, B via skywave and C via space wave (c) B and C are transmitted via ground wave...
In the circuit, find the value of RC.
Answer: Ie = Ic + Ib IcRc + Vce + IeRe = Vcc Rib + Vbe + IeRe = Vcc Ib = 11.5/200 mA Rc + Re = 1.56 kilo ohm Rc = 560 Ohm
For the transistor circuit shown in Fig.14.19, evaluate VE, RB, RE given IC = 1 mA, VCE = 3V, VBE = 0.5 V and VCC = 12 V, β = 100.
Answer: $ {{I}_{C}}={{I}_{B}}+{{I}_{E}} $ $ {{I}_{B}}<<<{{I}_{C}} $ $ \therefore {{I}_{C}}={{I}_{E}} $ $ {{I}_{C}}=1mA(given) $ $ \therefore {{I}_{C}}={{I}_{E}}=1mA $ By Kirchhoff's loop...
Consider a box with three terminals on top of it:
Three components namely, two germanium diodes and one resistor are connected across these three terminals in some arrangement. A student performs an experiment in which any two of these three...
An X-OR gate has the following truth table:
A B Y 0 0 0 0 1 1 1 0 1 1 1 0 It is represented by the following logic relation Build this gate using AND, OR, and NOT gates. Answer: In given logic relation Y = AˉB + ABˉ =Y1Y2 Now, the logic...
Suppose a ‘n’-type wafer is created by doping Si crystal having 5 × 1028 atoms/m3 with 1ppm concentration of As. On the surface 200 ppm Boron is added to create the ‘P’ region in this wafer. Considering ni = 1.5 × 1016 m–3,
(i) Calculate the densities of the charge carriers in the n & p regions. (ii) Comment which charge carriers would contribute largely for the reverse saturation current when the diode is reverse...
An electron (mass m) with an initial velocity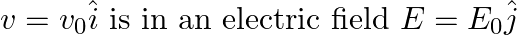 . If
. If  =
= 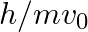 , it’s de Broglie wavelength at time t is given bya)
, it’s de Broglie wavelength at time t is given bya) 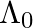 b)
b) 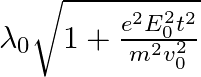 c)
c) 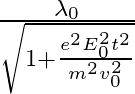 d )
d ) 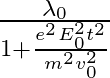
The correct answer is c)$ \frac{\lambda_{0}}{\sqrt{1+\frac{e^{2} E_{0}^{2} t^{2}}{m^{2} v_{0}^{2}}}} $ The de Broglie equation h=mv describes the relationship between a moving particle's momentum...
An electron (mass m ) with an initial velocity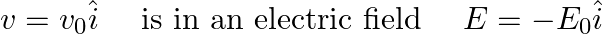 It’s de Broglie wavelength at time t is given bya)
It’s de Broglie wavelength at time t is given bya) 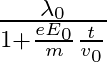 b)
b) 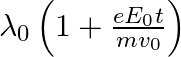 c)
c) 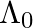 d)
d) 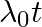
The correct answer is a) $\frac{\lambda_{0}}{1+\frac{e E_{0}}{m} \frac{t}{v_{0}}}$ The wave associated with moving particle is called matter wave or de-Broglie wave and it propagates in the form of...
Compound ‘A’ with molecular formula C4H9Br is treated with aq. KOH solution. The rate of this reaction depends upon the concentration of the compound ‘A’ only. When another optically active isomer ‘B’ of this compound was treated with aq. KOH solution, the rate of reaction was found to be dependent on the concentration of compound and KOH both. (i) Write down the structural formula of both compounds ‘A’ and ‘B’. (ii) Out of these two compounds, which one will be converted to the product with an inverted configuration.
Because the rate of reaction of compound ‘A' (C4H9Br) with aqueous KOH is solely determined by the concentration of compound ‘A,' the reaction happens via the SN1 mechanism, and product ‘A' is...
Classify the following compounds as primary, secondary and tertiary halides. (i) 1-Bromobut-2-ene (ii) 4-Bromopent-2-ene (iii) 2-Bromo-2-methylpropane
(i)A main halide is 1-bromobut-2-ene. (ii) Bromine is linked to the secondary carbon atom in 4-Bromopent-2-ene, making it a secondary halide. (iii) Bromine is linked to the tertiary carbon atom in...
Draw other resonance structures related to the following structure and find out whether the functional group present in the molecule is ortho, para directing or meta directing.
Because electron density is higher at ortho and para locations, the functional groups contained in these compounds are ortho-para directed.
Which of the products will be a major product in the reaction given below? Explain. CH3CH=CH2 + HI → CH3CH2CH2I + CH3CHICH30 (A) (B)
The molecule (B) will be the reaction's main product. This addition reaction is carried out according to Markovnikoff's rule, in which the hydrogen from the hydrogen halide is added to the carbon...
Which of the following compounds (a) and (b) will not react with a mixture of NaBr and H2SO4. Explain why?
Br2 gas is produced when NaBr and H2SO4 are combined. Because of the stable molecule created as a result of resonance stabilisation, molecule (b) will not react with Br2 gas.
Out of o-and p-bromobenzene which one has a higher melting point and why?
Because the symmetry of p-dibromobenzene allows the molecule to fit better in a crystal lattice, it has a higher melting point. As a result, breaking the bonds between the molecules needs a greater...
Which of the following compounds can be classified as aryl halides? (i) p-ClC6H4CH2CH(CH3)2 (ii) p-CH3CHCl(C6H4)CH2CH3 (iii) o-BrH2C-C6H4CH(CH3)CH2CH3 (iv) C6H5-Cl
Option (i) and (iv) are the answers. Because the halogen atom is directly linked to the aromatic ring in compounds (i) and (iv), these compounds are classed as aryl halides.
Which of the following compounds are gem-dihalides? (i) Ethylidene chloride (ii) Ethylene dichloride (iii) Methylene chloride (iv) Benzyl chloride
Option (i) and (iii) are the answers. Dihalides with two halogen atoms linked to the same carbon atom are known as gem-dihalides. Gem-dihalides are formed when two halogen atoms are present on the...
Which of the following statements are correct about the kinetics of this reaction? (i) The rate of reaction depends on the concentration of only (b). (ii) The rate of reaction depends on the concentration of both (a) and (b). (iii) Molecularity of reaction is one. (iv) Molecularity of reaction is two.
Option (i) and (iii) are the answers. The SN1 mechanism is used in the given reaction. The production of carbocation is a gradual process in the SN1 mechanism. As a result, the pace of reaction is...
arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.(i) (a) < (b) < (c) (ii) (b) < (a) < (c) (iii) (c) < (b) < (a) (iv) (a) < (c) < (b)
Option (iii) is the answer. The amount of electron releasing groups increases the reactivity of aryl halides; the fewer the electron releasing groups, the slower the rate of nucleophilic...
Which of the following compounds will give racemic mixture on nucleophilic substitution by OH– ion?(i) (a) (ii) (a), (b), (c) (iii) (b), (c) (iv) (a), (c)
Option (i) is the answer. At least one chiral carbon must be present in the molecule to display racemic mixing. Chiral carbon is an asymmetric carbon that is linked to four distinct sorts of atoms...
Reaction of C6H5CH2Br with aqueous sodium hydroxide follows ____________. (i) SN1 mechanism (ii) SN2 mechanism (iii) Any of the above two depending upon the temperature of the reaction (iv) Saytzeff rule
Option (i) is the answer. The $S_N$ 1 mechanism operates in watery media. As a result of this reaction, a stable carbocation forms as an intermediate.
Molecules whose mirror image is non-superimposable over them are known as chiral. Which of the following molecules is chiral? (i) 2-Bromobutane (ii) 1-Bromobutane (iii) 2-Bromopropane (iv) 2-Bromopropan-2-ol
Chiral molecules are those that lack a plane of symmetry as well as a centre of symmetry.. It is a chiral molecule because it lacks the plane of symmetry and the centre of symmetry. Option (i) is...
What should be the correct IUPAC name for diethylbromomethane? (i) 1-Bromo-1,1-diethylmethane (ii) 3-Bromopentane (iii) 1-Bromo-1-ethylpropane (iv) 1-Bromopentane
Option (ii) is the answer. Diethylbromomethane's proper IUPAC designation is 3 - Bromopentane. $C_5H_{11}Br$ is the chemical formula for it.
Which is the correct IUPAC name for? (i) 1-Bromo-2-ethylpropane (ii) 1-Bromo-2-ethyl-2-methylethane (iii) 1-Bromo-2-methylbutane (iv) 2-Methyl-1-bromobutane
Option (iii) is the answer. correct IUPAC name for given compound is 1-Bromo-2-methylbutane
AB is a diameter of a circle and C is any point on the circle. Show that the area of Δ ABC is maximum, when it is isosceles.
Let consider AB be the width and C is any point on the circle with range r. \[\angle ACB\text{ }=\text{ }90o\] [angle in the semi-circle is 90o] Let \[AC\text{ }=\text{ }x\] Squaring on both the...
If the sum of the surface areas of cube and a sphere is constant, what is the ratio of an edge of the cube to the diameter of the sphere, when the sum of their volumes is minimum?
We should accept x be to the edge and r be the span of the circle. Surface space of 3D square \[=\text{ }6x2\] Also, surface space of the circle \[=\text{ }4\pi r2\] Presently, their total is...
The order of reactivity of following alcohols with halogen acids is ___________.(i) (A) > (B) > (C) (ii) (C) > (B) > (A) (iii) (B) > (A) > (C) (iv) (A) > (C) > (B)
Option (ii) is the answer. Alcohol reactivity towards halogen acids diminishes in the following order: (C) > (B) > (A) Because tertiary carbocation is the most stable of the three, it is...
Find the dimensions of the rectangle of perimeter 36 cm which will sweep out a volume as large as possible, when revolved about one of its sides. Also find the maximum volume. Solution:
We should believe x and y to be the length and expansiveness of given square shape ABCD. As per the inquiry, the square shape will be settled with regards to side AD which making a chamber with...
An open box with square base is to be made of a given quantity of card board of area c2. Show that the maximum volume of the box is c3/ 6√3 cubic units.
Leave x alone the length of the side of the square base of the cubical open box and y be its height Along these lines, the surface space of the open box
If the straight-line x cos α + y sin α = p touches the curve x2/a2 + y2/b2 = 1, then prove that a2 cos2 α + b2 sin2 α = p2.
The given bend is \[\mathbf{x2}/\mathbf{a2}\text{ }+\text{ }\mathbf{y2}/\mathbf{b2}\text{ }=\text{ }\mathbf{1}\] and the straight-line \[\mathbf{x}\text{ }\mathbf{cos}\text{ }\mathbf{\alpha }\text{...
A telephone company in a town has 500 subscribers on its list and collects fixed charges of Rs 300/- per subscriber per year. The company proposes to increase the annual subscription and it is believed that for every increase of Rs 1/- one subscriber will discontinue the service. Find what increase will bring maximum profit?
We should consider that the organization expands the yearly membership by \[\mathbf{Rs}\text{ }\mathbf{x}.\] Along these lines, x is the quantity of supporters who end the administrations. ...
Find the points of local maxima, local minima and the points of inflection of the function f (x) = x5 – 5×4 + 5×3 – 1. Also find the corresponding local maximum and local minimum values.
Given, \[\mathbf{f}\text{ }\left( \mathbf{x} \right)\text{ }=\text{ }\mathbf{x5}\text{ }\text{ }\mathbf{5x4}\text{ }+\text{ }\mathbf{5x3}\text{ }\text{ }\mathbf{1}\] Separating the capacity,...
Which of the following are benzylic alcohols?
Option (ii) and (iii) are the answers. Benzylic alcohols, as we all know, are molecules with an alcohol functional group on a carbon atom that is directly linked to the Benzene ring. Because the –OH...
Prove that f (x) = sin x + √3 cos x has maximum value at x = π/6.
Let ∆ABC be the right-angled triangle in which \[\angle B\text{ }=\text{ }{{90}^{o}}\] Let \[\mathbf{AC}\text{ }=\text{ }\mathbf{x},\text{ }\mathbf{BC}\text{ }=\text{ }\mathbf{y}\] In this way,...
Arrange the following compounds in increasing order of boiling point. Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol (i) Propan-1-ol, butan-2-ol, butan-1-ol, pentan-1-ol (ii) Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol (iii) Pentan-1-ol, butan-2-ol, butan-1-ol, propan-1-ol (iv) Pentan-1-ol, butan-1-ol, butan-2-ol, propan-1-ol
Option (i) is the answer As the moleculer mass of the alcohol grows, the boiling point rises. Furthermore, $1^0$ alcohols have greater boiling points than $2^0$ alcohols among isomeric alcohols.
Prove that f (x) = sin x + √3 cos x has maximum value at x = π/6.
ACCORDING TO QUES, HENCE FUNCTION has maximum value at \[x\text{ }=\text{ }\pi /6\] and maximum value is \[2.\]
How will you distinguish between the dispersed phase and dispersion medium in an emulsion?
Emulsion is the dispersion of one liquid into another invisible liquid. The dispersed phase is always available in lesser amount when compared to the dispersion medium.
At what point, the slope of the curve y = – x3 + 3×2 + 9x – 27 is maximum? Also find the maximum slope.
Given, CURVE \[y\text{ }=\text{ }\text{ }x3\text{ }+\text{ }3x2\text{ }+\text{ }9x\text{ }\text{ }27\] Separating the two sides w.r.t. x, we get \[dy/dx\text{ }=\text{ }-\text{ }3x2\text{ }+\text{...
Based on Hardy-Schulze rule explain why the coagulating power of phosphate is higher than chloride.
According to Hardy-Schulze law, electrolyte coagulation properties depend on the valency of coagulation ion. The higher the charge in flocculating ion the smaller the amount of electrolyte required...
Why does the bleeding stop by rubbing moist alum?
Moist alum is highly charged with Al3+ and SO42-ions which activate the charged protein molecules present in the blood. This causes the blood protein to coagulate and stops bleeding.
Which of the following compounds will react with sodium hydroxide solution in water? (i) C6H5OH (ii) C6H5CH2OH (iii) (CH3)3 COH (iv) C2H5OH
Option (i) is the answer Because phenols are more acidic than alcohols, they will react with a sodium hydroxide solution in water.
Show that f(x) = tan–1(sin x + cos x) is an increasing function in (0, π/4).
Given, \[f\left( x \right)\text{ }=\text{ }tan1\left( sin\text{ }x\text{ }+\text{ }cos\text{ }x \right)\text{ }in\text{ }\left( 0,\text{ }\pi /4 \right).\] DIFFERENTIATING the two sides w.r.t. x, we...
How does the precipitation of colloidal smoke take place in Cottrell precipitator?
The Cottrell precipitator is a filtering tool that removes fine particles such as dust and fumes from flowing electricity. It neutralizes the charge on carbon particles.
Why does leather get hardened after tanning?
When the skin is soaked in tannin, coagulation occurs as a result of a combination of animal skin (positively charged) and tannin (negatively charged). Thus resulting in the hardening of the leather...
Why are some medicines more effective in the colloidal form?
The colloidal solution has a larger surface area than the true solution. The surface area is directly proportional to the level of assimilation in our body. So, the drugs work effectively when...
How do emulsifying agents stabilise the emulsion?
1. Emulsifying agents stabilize the emulsion by forming interfacial film between the suspended particles and the dispersion medium. 2. Example: Agar (used in foods)
A colloid is formed by adding FeCl3 in excess of hot water. What will happen if excess sodium chloride is added to this colloid?
FeCl3 when dissolved in hot water, forms hydrated ferric oxide, a sol which is positively charged due to the absorption of Fe3+ ions. By adding NaCl, the negatively charged chloride ions reduce the...
Show that for a ³ 1, f (x) = √3 sin x – cos x – 2ax + b is decreasing in R.
ACCORDING TO QUES, \[f~\left( x \right)\text{ }=\text{ }\surd 3\text{ }sin~x\text{ }~cos~x\text{ }~2ax\text{ }+\text{ }b,\text{ }a~{}^\text{3}\text{ }1\] differentiating both sides w.r.t. x WE HAVE...
What causes Brownian motion in colloidal dispersion?
1. The Brownian motion occurs because of the collision between the colloidal particles and dispersion medium. 2. The movement causes the stability of the colloidal solution.
Show that f (x) = 2x + cot-1 x + log [√(1 + x2) – x] is increasing in R.
Given, \[f\text{ }\left( x \right)\text{ }=\text{ }2x\text{ }+\text{ }bed\text{ }1\text{ }x\text{ }+\text{ }log\text{ }\left[ \surd \left( 1\text{ }+\text{ }x2 \right)\text{ }\text{ }x...
What happens when the electric field is applied to a colloidal solution?
When an electric field is applied to a colloidal solution, the colloidal particles move to different electrodes depending on their charge.
Why do we add alum to purify water?
1. Alum is basically the potassium aluminum sulphate [KAl(SO4)2]. 2. When dissolved in alkaline bicarbonate it forms a gelatinous precipitate. 3. When added to water it attracts colloidal particles...
What is collodion?
Collodion: 1. Collodion is a 4% solution of nitrocellulose prepared in a mixture of alcohol and ether. 2. Collodion is a solution which is syrupy in texture and can be inflammable.
Gelatin which is a peptide is added in icecreams. What can be its role?
Gelatin acts as an emulsifying agent for hydrophilic colloid. They absorb water content from ice cream and are also a good source of protein. They also give a soft texture and a glossy appearance to...
Show that the line x/a + y/b = 1, touches the curve y = b . e-x/a at the point where the curve intersects the axis of y.
Given curve condition, \[y\text{ }=\text{ }b\text{ }.\text{ }e-x/an\] and line condition \[x/a\text{ }+\text{ }y/b\text{ }=\text{ }1\] Presently, let the directions of where the curve meets the...
How many alcohols with molecular formula C4H10O are chiral? (i) 1 (ii) 2 (iii) 3 (iv) 4
Option (i) is the answer. Alcohols with the chemical formula $C_4H_{10}O$ can have a variety of structures. A similar chemical formula may be used to make a total of four alcohols. Butan-1-ol,...
At what points on the curve x2 + y2 – 2x – 4y + 1 = 0, the tangents are parallel to the y-axis?
Given, the condition of the bend is \[x2\text{ }+\text{ }y2\text{ }\text{ }2x\text{ }\text{ }4y\text{ }+\text{ }1\text{ }=\text{ }0\text{ }\ldots \text{ }..\text{ }\left( I \right)\] Separating both...
Find the equation of the normal lines to the curve 3×2 – y2 = 8 which are parallel to the line x + 3y = 4
Given curve, \[3x2\text{ }\text{ }y2\text{ }=\text{ }8\] Separating the two sides w.r.t. x, we get \[6x\text{ }\text{ }2y.\text{ }dy/dx\text{ }=\text{ }0\Rightarrow -\text{ }2y\left( dy/dx...
