(a) Assertion and Reason both are true, Reason is the correct explanation of Assertion. (b) Assertion and Reason both are true but Reason is not the correct explanation of Assertion. (c) Assertion...
Match the compounds given in Column I with the oxidation state of cobalt present in it (given in Column II) and assign the correct code. with the oxidation state of cobalt present in it (given in Column II) and assign the correct code.
Solution: \[\left( c \right)\text{ }\left( A\text{ }\to \text{ }5 \right),\text{ }\left( B\text{ }\to \text{ }1 \right),\text{ }\left( C\text{ }\to \text{ }4 \right),\text{ }\left( D\text{...
Match the complex species given in Column I with the possible isomerism given in Column II and assign the correct code:
Solution: \[\left( d \right)\text{ }\left( A\text{ }\to 4 \right),\text{ }\left( B\to \text{ }1 \right),\text{ }\left( C\text{ }\to 2 \right),\text{ }\left( D\text{ }\to 3 \right)\] Isomerism...
Rewrite each of the following statements in the form of conditional statements
(i) The square of an odd number is odd.
(ii) You will get a sweet dish after the dinner.
Solution: (i) In the form of conditional statement, expression is If $p$, then $q$ So now, In the statement $p$ and $q$ are $p$: The number is odd. $q$: The square of odd number is odd. As a result,...
Match the complex ions given in Column I with the hybridisation and number of unpaired electrons given in Column II and assign the correct code :
Solution: \[\left( a \right)\text{ }\left( A\text{ }\to \text{ }3 \right),\text{ }\left( B\text{ }\to 1 \right),\text{ }\left( C\text{ }\to \text{ }5 \right),\text{ }\left( D\to \text{ }2...
Write down the negation of following compound statements
(i) |x| is equal to either x or – x.
(ii) 6 is divisible by 2 and 3.
Solution: (i) The given statement is compound statement then components are, $P$: $\mid{x}\mid$ is equal to $x$. $\sim p$: $\mid{x}\mid$ is not equal to $x$. $q$: $\mid{x}\mid$ is equal to $–x$....
Match the coordination compounds given in Column I with the central metal atoms given in Column II and assign the correct code : Code : (i) A (5) B (4) C (1) D (2) (ii) A (3) B (4) C (5) D (1) (iii) A (4) B (3) C (2) D (1) (iv) A (3) B (4) C (1) D (2)
Solution: \[\left( b \right)\text{ }\left( A\text{ }\to 5 \right),\text{ }\left( B\text{ }\to \text{ }4 \right),\text{ }\left( C\text{ }\to \text{ }1 \right),\text{ }\left( D~\to 2 \right)\] ...
Write down the negation of following compound statements
(i) 35 is a prime number or a composite number.
(ii) All prime integers are either even or odd.
Solution: (i) The statement given is compound statement whose components are, $P$: 35 is a prime number $\sim p$: 35 is not a prime number. $q$: 35 is a composite number $\sim q$: 35 is not a...
Write down the negation of following compound statements
(i) 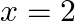 and
and 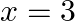 are the roots of Quadratic equation
are the roots of Quadratic equation 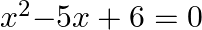 .
.
(ii) A triangle has either 3-sides or 4-sides.
Solution: (i) The sentence given is a compound statement whose components are $p$: $x = 2$ is a root of the Quadratic equation $x^{2}{-}5x + 6 = 0$. $\sim p$: $x = 2$ is not a root of the Quadratic...
Match the complex ions given in Column I with the colours given in Column II and assign the correct code :
Solution: \[\left( b \right)\text{ }\left( A\text{ }\to 4 \right),\text{ }\left( B\text{ }\to \text{ }3 \right),\text{ }\left( C\text{ }\to \text{ }2 \right),\text{ }\left( D\text{ }\to \text{ }1...
Write down the negation of following compound statements
(i) All rational numbers are real and complex.
(ii) All real numbers are rationals or irrationals.
Solution: (i) The statement given is compound statement whose components are, $P$: All rational numbers are real. $\sim p$: All rational numbers are not real. $q$: All rational numbers are complex....
Name the type of isomerism when ambidentate ligands are attached to a central metal ion. Give two examples of ambidentate ligands.
Solution: Ambidendate ligands are those having diverse two restricting destinations. Example: Isothiocyanato Thiocyanato and Nitrite-N Nitrito-O The kind of isomerism when ambidentate ligands are...
Translate the following statements into symbolic form
(i) Students can take Hindi or English as an optional paper.
Solution: (i) The sentence given is a compound statement whose components are $p$: Hindi is the optional paper $q$: English is the optional paper It can now be represented in symbolic function as,...
CuSO4.5H2O is blue while CuSO4 is colourless. Why?
Solution: In CuSO4.5H2O, there are water particles that go about as ligands. The electrons will invigorate to higher d orbital and show tone. While, in CuSO4, there are no water particles to go...
Translate the following statements into symbolic form
(i) A number is either divisible by 2 or 3.
(ii) Either x = 2 or x = 3 is a root of 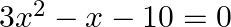
Solution: (i) The sentence given is a compound statement whose components are $p$: A number is divisible by 2 $q$: A number is divisible by 3 It can now be represented in symbolic function as,...
Give the electronic configuration of the following complexes based on Crystal Field Splitting theory. [CoF6]3–, [Fe(CN)6]4– and [Cu(NH3)6]2+.
Solution: \[{{[CO{{F}_{\mathbf{6}}}]}^{\mathbf{3}-}}:\text{ }C{{o}^{\mathbf{3}+}}~({{d}^{\mathbf{6}}})\]
Translate the following statements into symbolic form
(i) 2, 3 and 6 are factors of 12.
(ii) Either x or x + 1 is an odd integer.
Solution: (i) The sentence given is a compound statement whose components are $p$: 2 is a factor of 12 $q$: 3 is a factor of 12 $r$: 6 is a factor of 12 It can can be represented in symbolic...
Which of the following complexes show linkage isomerism? (a) [CO(NH3)5(NO2)]2+ (b) [CO(H2O)5CO]3+ (c) [Cr(NH3)5SCN]2+ (d) [Fe(en)2Cl2]+
Solution: (a, c) NO2 and SCN are ambidentate ligands subsequently, show linkage isomerism.
Identify the correct statements for the behaviour of ethane-1, 2-diamine as a ligand. (a) It is a neutral ligand (b) It is a didentate ligand (c) It is a chelating ligand (d) It is a unidentate ligand
Solution: hence, option a, b and c are correct
Identify the optically active compounds from the following: (a) [Co(en)3]3+ (b) trans-[Co(en)2Cl2]+ (c) cis-[Co(en)2Cl2]+ (d) [Cr(NH3)5Cl]
Solution:
Translate the following statements into symbolic form
(i) Rahul passed in Hindi and English.
(ii) x and y are even integers.
Solution: (i) The sentence given here is a compound statement whose components are $p$: Rahul passed in Hindi $q$: Rahul passed in English It can now be represented in symbolic function as, $p\wedge...
Which of the following complexes are heteroleptic? (a) [Cr(NH3)6]3+ (b) [Fe(NH3)4 Cl2]+ (c) [Mn(CN)6]4– (d) [Co(NH3)4Cl2]
Solution: (b, d) In complexes, [Fe(NH3)4Cl2]+ and [CO(NH3)4Cl2], metal is bonded to more than one sort of ligands subsequently, they are heteroleptic.
Which of the following complexes is homoleptic? (a) [Co(NH3)6]3+ (b) [Co(NH3)4 Cl2]+ (c) [Ni(CN)4]2– (d) [Ni(NH3)4Cl2]
Solution: (a, c) In complexes [Co(NH3)6]3+ and [Ni(CN)4]2-, both Co and Ni are connected to one sort of ligands just subsequently, they are homoleptic. hence, option a and c are correct
An aqueous pink solution of cobalt(II) chloride changes to deep blue on the addition of an excess of HCl. This is because____________. (a) [Co(H2O)6]2+ is transformed into [CoCl6]4– (b) [Co(H2O)6]2+ is transformed into [CoCl4]2– (c) tetrahedral complexes have smaller crystal field splitting than octahedral complexes. (d) tetrahedral complexes have larger crystal field splitting than octahedral complex.
Solution: (b, c) Aqueous pink arrangement of cobalt (II) chloride is because of electronic progress of electron from t2g to eg energy level of [Co(H2O)6]2+ complex. At the point when overabundance...
Which of the following options are correct for [Fe(CN)6]3- complex? (a) d2sp3 hybridisation (b) sp3d2 hybridisation (c) Paramagnetic (d) Diamagnetic
Solution: hence, option a and c are correct for [Fe(CN)6]3- complex
Atomic number of Mn, Fe, Co and Ni are 25, 26 27 and 28 respectively. Which of the following outer orbital octahedral complexes have same number of unpaired electrons? (a) [MnCl6]3- (b) [FeF6]3- (c) [CoF6]3- (d) [Ni(NH3)6]2+
Solution: so, option a and c are the correct answer
The atomic number of Mn, Fe and Co are 25, 26 and 27 respectively. Which of the following inner orbital octahedral complexions are diamagnetic? (a) [Co(NH3)6]3+ (b) [Mn(CN)6]3– (c) [Fe(CN)6]4– (d) [Fe(CN)6]3–
Solution: hence , a and c are the correct answer
IUPAC name of [Pt(NH3)2Cl(NO2)] is (a) platinum diaminechloronitrite (b) chloronitrito-N-ammineplatinum (II) (c) diamminechloridonitrito-N-platinum (II) (d) diamminechloronitrito-N-platinate (II).
Solution: (c) [Pt(NH3)2Cl(NO2)] is diamminechloridonitrito-N-platinum (II)
What kind of isomerism exists between [Cr(H2O)6]Cl3 (violet) and [Cr(H2O)5Cl)Cl2.H2O (greyish- green)? (a) Linkage isomerism (b) Solvate isomerism (c) Ionisation isomerism (d) Coordination isomerism
Solution: (b) The given mixtures have diverse number of water atoms inside and outside the organize circle.
Which of the following species is not expected to be a ligand? (a) NO (b) NH4– (c) NH2CH2CH2NH2 (d) CO
Solution: (b) Ligand should give a pair of electrons or inexactly held electron pair to metal and shape a M – L bond,
A chelating agent has two or more than two donor atoms to bind to a single metal ion. Which of the following is not a chelating agent? (a) Thiosulphate (b) Oxalato (c) Glycinato (d) Ethane-1, 2-diamine
Solution: (a) Thiosulphate or S2O3–isn't a chelating specialist since it is a monodentate ligand.
The compounds [Co(SO4)(NH3)5]Br and [CO(SO4)(NH3)5]Cl represent (a) linkage isomerism (b) ionization isomerism (c) coordination isomerism (d) no isomerism.
Solution: (d) [Co(SO4)(NH3)5]Br and [CO(SO4)(NH3)5]Cl address no isomerism since they are different compounds.
Due to the presence of ambidentate ligands coordination compounds show isomerism. Palladium complexes of the type [Pd(C6H5)2(SCN)2] and [Pd(C6H5)2(NCS)2] are (a) linkage isomers (b) coordination isomers (c) ionisation isomers (d) geometrical isomers
Solution: (a) The ligands having two distinctive holding locales are known as ambident ligands e.g., NCS, NO2, and so forth Here, NCS has two restricting locales at N and S. Subsequently, NCS...
The CFSE for octahedral [CoCl6]4– is 18,000 cm–1. The CFSE for tetrahedral [CoCl4]2– will be (a) 18,000 cm–1 (b) 16,000 cm–1 (c) 8,000 cm–1 (d) 20,000 cm–1
Solution:
Indicate the complex ion which shows geometrical isomerism. (a) [Cr(H2O)4Cl2]+ (b) [Pt(NH3)3 Cl] (c) [Co(NH3)6]3+ (d) [Co(CN)5(NC)]3–
Solution:
The stabilisation of coordination compounds due to chelation is called the chelate effect. Which of the following is the most stable complex species? (a) [Fe(CO)5] (b) [Fe(CN)6]3– (c) [Fe(C2O4)3]3– (d) [Fe(H2O)6]3+
Solution: (c) arrangement of cycle by linkage between metal particle and ligand balances out the coordination compound. The ligand which chelates the metal particle are known as chelating ligand....
The correct IUPAC name of [Pt(NH3)2Cl2] is (a) diamminedichloridoplatinum (II) (b) diamminedichloridoplatinum (IV) (c) diamminedichloridoplatinum (I) (d) dichloridodiammineplatinum (IV)
Solution: (a) [Pt(NH3)2Cl2] is diamminedichloridoplatinum (II) .
When 1 mol CrCl3.6H2O is treated with an excess of AgNO3, 3 mol of AgCl are obtained. The formula of the complex is : (a) [CrCl3 (H2O)3].3H2O (b) [CrCl2(H2O)4]Cl.2H2O (c) [CrCl(H2O)5]Cl2.H2O (d) [Cr(H2O)6]Cl3
Solution: (d) 3 mol of AgCl implies 3Cl are given in the arrangement henceforth, the equation of the complex will be [Cr(H2O)6]Cl3.
When 0.1 mol COCl3(NH3)5 is treated with excess of AgNO3, 0.2 mol of AgCl are obtained. The conductivity of solution will correspond to (a) 1:3 electrolyte (b) 1:2 electrolyte (c) 1:1 electrolyte (d) 3:1 electrolyte
Solution: (b) One mole of AgNO3 accelerates one mole of chloride particle. In the above response, when 0.1 mole COCl3(NH3)5 is treated with abundance of AgNO3, 0.2 mole of AgCl are obtained hence,...
The colour of the coordination compounds depends on the crystal field splitting. What will be the correct order of absorption of wavelength of light in the visible region, for the complexes, [Co(NH3)6]3+, [Co(CN)6]3–, [Co(H2O)6]3+ (a) [Co(CN)6]3–> [Co(NH3)6]3+>[Co(H2O)6]3+ (b) [Co(NH3)6]3+> [Co(H2O)6]3+> [Co(CN)6]3– (c) [Co(H2O)6]3+> [Co(NH3)6]3+> [Co(CN)6]3– (d) [Co(CN)6]3–> [Co(NH3)6]3+> [Co(H2O)6]3+
Solution:
Which of the following complexes formed by Cu2+ ions is most stable?
Solution: (b) The greater the value of log K, the more prominent will be strength of complicated compound shaped. For response, For this response, log K has most elevated worth among the...
If 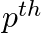 ,
, 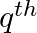 , and
, and 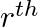 terms of an A.P. and G.P. are both a, b and c respectively, show that
terms of an A.P. and G.P. are both a, b and c respectively, show that 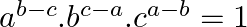
Solution: Let m be the first term of the AP and d be the common difference Let I be the GP’s first term and s be the common ration The Ap’s nth term is given as $t_{n}=a+{(n-1)}d$ in which the first...
If the sum of p terms of an A.P. is q and the sum of q terms is p, show that the sum of p + q terms is – (p + q). Also, find the sum of first p – q terms (p > q).
Solution: An AP’s sum of n terms is given by ${S_{n}}={\frac{n}{2}}(2a+(n-1)d)$ Where the first term is 'a' and the common difference is 'd'. It is given that $S_{p}=q$ and $S_{q}=p$ $\Rightarrow...
If 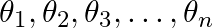 are in A.P., whose common difference is d, show that
are in A.P., whose common difference is d, show that 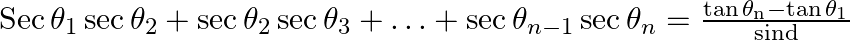
Solution: It is given that $\theta_{1}, \theta_{2}, \theta_{3}, \ldots, \theta_{n}$ are in the form of A.P., and $d$ is the common difference, We now need to prove that $\operatorname{Sec}...
If 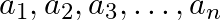 are in A.P., where
are in A.P., where 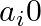 for all i, show that
for all i, show that 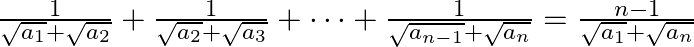
Solution: It is given that $a_{1}, a_{2}, a_{3}, \ldots, a_{n}$ are in the form of AP in which $a_{i}\lt{0}$ for all i. To prove that: $\begin{array}{l}...
Write the negation of the following simple statements
(i) Cow has four legs.
(ii) A leap year has 366 days.
(i) "Not p" is the negation of the assertion p. The negation of p is represented by $\sim p$. The truth value of $\sim p$ is the opposite of the truth value of p. The negation of the statement is...
Write the negation of the following simple statements
(i) Violets are blue.
(ii) √5 is a rational number.
(i) "Not p" is the negation of the assertion p. The negation of p is represented by $\sim p$. The truth value of $\sim p$ is the opposite of the truth value of p. The negation of the statement is...
Write the component statements of the following compound statements and check whether the compound statement is true or false.
(i) All living things have two eyes and two legs.
(ii) 2 is an even number and a prime number.
(i) A compound statement is made up of two or more statements (Components). As a result, the elements of the given sentence "All living things have two eyes and two legs" are as follows: p: All...
Write the component statements of the following compound statements and check whether the compound statement is true or false.
(i) 57 is divisible by 2 or 3.
(ii) 24 is a multiple of 4 and 6.
(i) A compound statement is made up of two or more statements (Components). As a result, the elements of the provided statement "57 is divisible by 2 or 3" are as follows: p: 57 is divisible by 2....
Find the component statements of the following compound statements.
A rectangle is a quadrilateral or a 5 – sided polygon.
A compound statement is made up of two or more statements (Components). As a result, the elements of the given statement "A rectangle is a quadrilateral or a 5-sided polygon" are as follows: p: A...
Find the value of k so that the function f is continuous at the indicated point:
So, \[7\text{ }=\text{ }2k\] \[k\text{ }=\text{ }7/2\text{ }=\text{ }3.5\] SO VALUE OF K IS \[3.5\]
Find the sum of the series 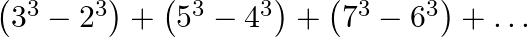 to (i)
to (i) 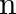 terms (ii) 10 terms
terms (ii) 10 terms
Solution: As per the question $\left(3^{3}-2^{3}\right)+\left(5^{3}-4^{3}\right)+\left(7^{3}-6^{3}\right)+\ldots$ Let's assume the series be...
If the 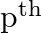 and
and 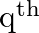 terms of a G.P. are
terms of a G.P. are 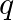 and
and 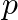 respectively, show that its
respectively, show that its 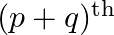 term is
term is 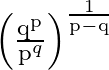
Solution: The $n^{\text {th }}$ term of Geometric Progression is given by $t_{n}=a r^{n-1}$ in which the first term is $a$ and the common difference is $r$ $\mathrm{p}^{\text {th }}$ term is given...
Find the component statements of the following compound statements.
(i) Plants use sunlight, water and carbon dioxide for photosynthesis.
(ii) Two lines in a plane either intersect at one point or they are parallel.
(i) A compound statement is made up of two or more statements (Components). As a result, the parts of the supplied statement "Plants use sunshine, water, and carbon dioxide for photosynthesis" are...
Find the component statements of the following compound statements.
(i) The number 100 is divisible by 3, 11 and 5.
(ii) Chandigarh is the capital of Haryana and U.P.
(i) A compound statement is made up of two or more statements (Components). As a result, the components of the provided statement "The number 100 is divisible by 3, 11, and 5" are as follows. p: 100...
The first term of an A.P.is a, and the sum of the first 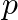 terms is zero, show that the sum of its next q terms is
terms is zero, show that the sum of its next q terms is 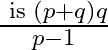 . [Hint: Required sum
. [Hint: Required sum ![Rendered by QuickLaTeX.com \left.=\mathrm{S}_{\mathrm{p}+\mathrm{q}}-\mathrm{S}_{\mathrm{p}}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-43a61029dbbcc74f00d776544ddd71be_l3.png)
Solution: It is given that the first term is ' $a$ ' and the sum of first '$p$' terms is $S_{p}=0$ We now need to find the sum of the next 'q' terms So, the total terms are $p+q$ As a result, Sum of...
Which of the following sentences are statements? Justify
Sum of opposite angles of a cyclic quadrilateral is 180°.
(ii) 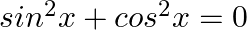
(i) If a statement is true or false but not both, it is a declarative sentence. "The sum of opposite angles of a cyclic quadrilateral is 180°," according to quadrilateral characteristics. As a...
Which of the following sentences are statements? Justify
(i) Where is your bag?
(ii) Every square is a rectangle.
(i) If a statement is true or false but not both, it is a declarative sentence. "Where is your bag?" is a question in this context. As a result, it is not a statement. (ii) Every square is a...
Which of the following sentences are statements? Justify
(i) 15 + 8 > 23.
(ii) y + 9 = 7.
(i) If a statement is true or false but not both, it is a declarative sentence. As a result, the expression "15 + 8 > 23" is incorrect. Because the L.H.S result will always equal the R.H.S...
Which of the following sentences are statements? Justify
(i) Sky is red.
(ii) Every set is an infinite set.
(i) If a statement is true or false but not both, it is a declarative sentence. The sentence "sky is red" is incorrect. As a result, the statement is false. (ii) If a statement is true or false but...
For what reason do compounds having comparable calculation have an alternate attractive second?
Solution: They vary in the quantity of combined and unpaired electrons. A solid field ligand will cause blending of electrons while a feeble field ligand won't cause matching. Blending or not...
Mastermind following complex particles in expanding request of precious stone field parting energy (DO) : [Cr(Cl)6]3–, [Cr(CN)6]3–, [Cr(NH3)6]3+.
Solution: The expanding request of gem field energy is [Cr(Cl)6]3–<[Cr(NH3)6]3+ <[Cr(CN)6]3– This is additionally the request for field strength of the ligands as indicated by the...
Clarify why [Fe(H2O)6]3+ has an attractive second worth of 5.92 BM while [Fe(CN)6]3–has a worth of just 1.74 BM.
Solution: For [Fe(H2O)6]3+, H2O is a powerless field ligand will not cause matching of electrons. Along these lines, the quantity of unpaired electrons will be 5. [Fe(CN)6]3–, Fe3+ has six unpaired...
Why are low twist tetrahedral edifices not framed?
Solution: For tetrahedral edifices, the gem field parting energy is excessively low. It is lower than blending energy in this way, the matching of electrons isn't supported and consequently the...
Find the area bounded by the curve y = sin x between x = 0 and x = 2p.
In view of gem field hypothesis clarify why Co(III) structures a paramagnetic octahedral complex with feeble field ligands though it frames a diamagnetic octahedral complex with solid field ligands.
Solution: The electronic design will be t42g e2g. It has 4 unpaired electron and paramagnetic. With weal ligand Δ0 < p. The design with solid field ligand will be t62g e0g. the Δ0 > p and...
Find the area bounded by the curve y = √x , x = 2y + 3 in the first quadrant and x-axis.
The curves are y = √x and line x = 2y + 3 Solving y = √x and x = 2y + 3, we get
The attractive snapshot of [MnCl4]2–is 5.92 BM. Clarify giving an explanation.
Solution: An attractive snapshot of 5.92 BM implies there are 5 unpaired electrons in light of the fact that \[Attractive\text{ }Moment\text{ }=\text{ }\surd \text{ }n\left( n+2 \right)\] The...
Find the area enclosed by the curve y = –x^2 and the straight-line x + y + 2 = 0.
The curve y = –x2 or x2 = –y and the line x + y + 2 = 0 Solving the two equation, we get \[x\text{ }-\text{ }{{x}^{2}}~+\text{ }2\text{ }=\text{ }0\] \[{{x}^{2}}~\text{ }-x\text{ }\text{ }-2\text{...
A complex of the kind [M(AA)2X2]n+is known to be optically dynamic. What does this demonstrate about the design of the complex? Give one illustration of such complicated.
Solution: The design must be cis-octahedral. Model for a particularly mind boggling is [Co(en)2Cl2]+ which is optically dynamic.
A coordination compound CrCl3.4H2O hastens silver chloride when treated with silver nitrate. The molar conductance of its answer relates to a sum of two particles. Compose the primary recipe of the compound and name it.
Solution: Assuming it structures silver chloride, there is without one chlorine iota outside the coordination circle. The primary recipe must be [Cr(H2O)4Cl2]Cl. The name of this complex is...
Organize the accompanying buildings in the expanding request of conductivity of their answer: [Co(NH3)3Cl3], [Co(NH3)4Cl2] Cl, [Co(NH3)6]Cl3 , [Cr(NH3)5Cl]Cl2
Solution: The expanding request of conductivity is as per the following: [Co(NH3)3Cl3]<[Co(NH3)4Cl2]Cl< [Cr(NH3)5Cl]Cl2<[Co(NH3)6]Cl3
Find the area of the region bounded by y = √x and y = x.
The equations of curve y = √x and line y = x Solving the equations y = √x ⇒ y2 = x and y = x, we get \[\begin{array}{*{35}{l}} {{x}^{2}}~=\text{ }x \\ {{x}^{2}}~-\text{ }x\text{ }=\text{ }0 \\...
Reactivity of change components diminishes routinely from Sc to Cu. Clarify.
Solution: Viable atomic charge increments as we move along the period from left to right. Because of the explanation, the size likewise lessens. Subsequently the electrons will be held all the more...
While topping off of electrons in the nuclear orbitals, the 4s orbital is filled before the 3d orbital yet the opposite occurs during the ionization of the molecule. Clarify why?
Solution: Electrons are filled by the n+l rule. Assuming an orbital has lower n+l esteem, the electron will enter that orbital. \[\begin{array}{*{35}{l}} For\text{ }3d,\text{ }n+l=\text{...
Determine the area under the curve y = √(a^2 – x^2) included between the lines x = 0 and x = a.
\[\begin{array}{*{35}{l}} y~=\text{ }\surd \left( {{a}^{2}}~-~{{x}^{2}} \right)\text{ }\Rightarrow ~{{y}^{2}}~=~{{a}^{2}}~-~{{x}^{2}} \\ {{x}^{2~}}+~{{y}^{2}}~=~{{a}^{2}} \\ \end{array}\] which is...
The halides of progress components become more covalent with expanding oxidation condition of the metal. Why?
Solution: Halides become more covalent with expanding oxidation state. As the oxidation state expands, the charge on the iota increments and the size of the particle of progress component...
E° of Cu is + 0.34V while that of Zn is – 0.76V. Clarify.
Solution: The decreased type of Cu2+ is more steady than the oxidized type of Cu. In this manner, the worth of E° is positive for Cu. Eliminating two electrons gives a steady setup [Ar]3d10 with...
An answer of KMnO4 on decrease yields either lackluster arrangement or an earthy colored encourage or a green arrangement relying upon the pH of the arrangement. What various phases of the decrease do these address and how are they completed?
Solution: In acidic medium, permanganate changes to manganous particle which is lackluster. \[MnO4-+8H+\text{ }+\text{ }5e-\to \text{ }Mn2+\text{ }+\text{ }4H2O\] (drab) In basic...
Draw a rough sketch of the curve y = √(x – 1) in the interval [1, 5]. Find the area under the curve and between the lines x = 1 and x = 5.
The curve is \[\begin{array}{*{35}{l}} y~=\text{ }\surd \left( x~-\text{ }1 \right) \\ \Rightarrow \text{ }{{y}^{2}}~=\text{ }x\text{ }-\text{ }1 \\ \end{array}\] Plotting the curve and finding...
At the point when an orange arrangement containing Cr2O72–particle is treated with an antacid, a yellow arrangement is framed and when H+ particles are added to a yellow arrangement, an orange arrangement is acquired. Clarify for what reason does this occur?
Solution: At the point when Cr2O72–is treated with an antacid: \[\left( orange \right)\text{ }Cr2O72+\text{ }OH-\to \text{ }2CrO42-\left( yellow \right)\] At the point when the yellow...
Clarify why the shade of KMnO4 vanishes when oxalic corrosive is added to its answer in acidic medium.
Solution: This is a redox titration. The profound purple shade of KMnO4 vanishes because of the development of MnSO4. \[\begin{array}{*{35}{l}} ~ \\ 5H2C2O4\text{ }+\text{ }2KMnO4\text{...
Calculate the area under the curve y = 2 √x included between the lines x = 0 and x = 1.
Sketch the region {(x, 0) : y = √(4 – x^2)} and x-axis. Find the area of the region using integration.
Given, {(x, 0) : \[\begin{array}{*{35}{l}} y~=\text{ }\surd \left( 4\text{ }~-{{x}^{2}} \right)\ \\ So,\text{ }{{y}^{2}}~=\text{ }4\text{ }-\text{ }{{x}^{2}} \\ {{x}^{2}}~+\text{...
At the point when an earthy colored compound of manganese (A) is treated with HCl it gives a gas (B). The gas taken in abundance responds with NH3 to give an unstable compound (C). Distinguish intensifies A, B and C.
Solution: At the point when earthy colored co pound of manganese (A) is treated with HCl it gives chlorine gas. \[MnO2\text{ }+\text{ }4HCl\text{ }\to \text{ }MnCl2\text{ }+\text{ }Cl2\text{...
Find the area of region bounded by the line x = 2 and the parabola y^2 = 8x
The equation of line x = 2 and parabola y2 = 8x Putting value of x in the other equation, we have \[\begin{array}{*{35}{l}} {{y}^{2}}~=\text{ }8\left( 2 \right) \\ {{y}^{2}}~=\text{ }16 \\...
At the point when Cu2+ particle is treated with KI, a white hasten is shaped. Clarify the response with the assistance of the compound condition.
Solution: \[~2Cu2+\text{ }+\text{ }4I-\to \text{ }Cu2I2\text{ }+\text{ }I2\] Cu2+ gets decreased to Cu+, and I–gets oxidized to I2.
Find the area of the region included between y^2 = 9x and y = x
The curves are y2 = 9x and y = x Solving the above equations, we have \[\begin{array}{*{35}{l}} {{x}^{2}}~=\text{ }9x\text{ }\Rightarrow \text{ }{{x}^{2}}~\text{ }-9x\text{ }=\text{ }0 \\ x\left(...
In spite of the fact that +3 is the trademark oxidation state for lanthanoids however cerium additionally shows +4 oxidation state in light of the fact that (a) it has variable ionization enthalpy (b) it tends to accomplish respectable gas design (c) it tends to accomplish f° arrangement (d) it looks like Pb4+
Solution: (b, c) Cerium shows +4 oxidation state likewise on the grounds that it tends to accomplish respectable gas setup and achieve f° design. Ce – 4f15d'6s2 (Ce4+–4f°)
Which of the accompanying won’t go about as oxidizing specialists? (a) CrO3 (b) MoO3(c) WO3 (d) CrO42-
Solution: (b, c) An animal types can go about as an oxidizing specialist just when metal is available in high oxidation state however lower oxidation state show strength. As higher oxidations...
Find the area of the region bounded by the curve y^2 = 4x and x^2 = 4y.
The curves are y2 = 4x … (i) and x2 = 4y … (ii) On solving the equations, we get From (ii), y = x2/4 Putting value of y in (i), we have \[\begin{array}{*{35}{l}} {{\left( {{x}^{2}}/4...
Change components structure double mixtures with incandescent lamp. Which of the accompanying components will frame MF3 type compounds? (a) Cr (b) Co (c) Cu (d) Ni
Solution: (a, b) Cr and Co structure MF3 kind of mixtures. The capacity of fluorine to balance out the most elevated oxidation state is because of higher grid energy in CoF3 and higher bond enthalpy...
Which of the accompanying particles show higher twist just attractive second worth? (a) Ti3+ (b) Mn2+ (c) Fe2+ (d) Co3+
Solution: (b, c) Mn2+ (3d5) and Fe2+ (3d6) have 5 and 4 unpaired electrons thus higher upsides of twist just attractive second when contrasted with Ti3+ (3d1) and Co2+ (3d7).
Find the area of the region bounded by the curve y = x^3 and y = x + 6 and x = 0.
The curves are y = x3, y = x + 6 and x = 0 On solving y = x3 and y = x + 6, we have \[\begin{array}{*{35}{l}} {{x}^{3}}~=\text{ }x\text{ }+\text{ }6 \\ {{x}^{3}}~\text{ }-x\text{ }-\text{ }6\text{...
Find the area of the region bounded by the curves y^2 = 9x, y = 3x.
Given curves are y2 = 9x and y = 3x solving the two equations we have \[\begin{array}{*{35}{l}} {{\left( 3x \right)}^{2}}~=\text{ }9x \\ 9{{x}^{2}}~=\text{ }9x \\ 9{{x}^{2}}~\text{ }-9x\text{...
Which of the accompanying lanthanoids show +2 oxidation state other than the trademark oxidation state +3 of lanthanoids? (a) Ce (b) Eu (c) Yb (d) Ho
Solution: (b, c)
General electronic arrangement of actinoids is (n – 2)f1-14 (n – 1 )d0-2 ns2. Which of the accompanying actinoids have one electron in 6d orbital? (a) U (Atomic no. 92) (b) Np (Atomic no. 93) (c) Pu (Atomic no. 94) (d) Am (Atomic no. 95)
Solution:
Which of the accompanying actinoids show oxidation states up to +7? (a) Am (b) Pu (c) U (d) Np
Solution: (b, d) Np and Pu show +7 oxidation state.
solve the following:
Solution:
As dichromate, Cr (VI) is a solid oxidizing specialist in acidic medium however, Mo (VI) in MoO3 and W (VI) in WO3 are not on the grounds that (a) Cr (VI) is more steady than Mo(VI) and W(VI) (b) Mo(VI) and W(VI) are more steady than Cr(VI) (c) higher oxidation conditions of heavier individuals from bunch 6 of change series are more steady (d) lower oxidation conditions of heavier individuals from bunch 6 of change series are more steady
Solution: (b, c) In d-block components, for heavier components, the higher oxidation states are more steady. Thus, Mo(VI) and W(VI) are more steady than Cr (VI). That is the reason, Cr (VI) as...
Change components show attractive second because of twist and orbital movement of electrons. Which of the accompanying metallic particles have practically same twist just attractive second? (a) Co2+ (b) Cr2+ (c) Mn2+ (d) Cr3+
Solution: (a, d) Co2+ (3d7) and Cr3+ (3d3) have 3 unpaired electrons. Thus they have practically same twist just attractive second.
solve the following:
Solution:
solve the following:
Solution:
For the most part, progress components and their salts are shaded because of the presence of unpaired electrons in metal particles. Which of the accompanying mixtures are hued? (a) kMnO4 (b) Ce(SO4)2 (c) TiCl4 (d) Cu2Cl2
Solution: (a, b) KMnO4 and Ce(S04)2 are shaded because of charge move
For what reason is HCl not used to make the medium acidic in oxidation responses of KMnO4 in acidic medium? (a) Both HCl and KMn04 go about as oxidizing specialists. (b) KMnO4 oxidizes HCl into Cl2 which is likewise an oxidizing specialist. (c) KMnO4 is a more fragile oxidizing specialist than HCl. (d) KMnO4 goes about as a decreasing specialist within the sight of HCl.
Solution: (b) HCl isn't utilized to make the medium acidic in oxidation responses of KMnO4 in acidic medium. The explanation is that in case HCl is utilized, the oxygen delivered from KMnO4 + HCl is...
solve the following:
Solution:
In spite of the fact that zirconium has a place with 4d change series and hafnium to 5d change series and still, after all that they show comparative physical and substance properties in light of the fact that (a) both have a place with d-block (b) both have same number of electrons (c) both have comparative nuclear span (d) both have a place with a similar gathering of the intermittent table
Solution: (c) Zirconium and hafnium have comparable nuclear range thus they show comparable physical and synthetic properties.
solve the following:
Solution:
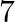 audio cassettes and
audio cassettes and 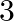 videocassettes cost
videocassettes cost 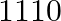 , while
, while 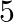 audio cassettes and
audio cassettes and 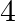 videocassettes cost
videocassettes cost 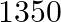 . Find the cost of audio cassettes and a video cassette.
. Find the cost of audio cassettes and a video cassette.
Let’s assume the cost of an audio cassette and that of a video cassette be ₹a and ₹b, respectively. Then forming equations according to the question, we have $7a+3b=1110$…(a) $5a+4b=1350$… (b) On...
Most elevated oxidation condition of manganese in fluoride is +4 (MnF4) however most noteworthy oxidation state in oxides is +7 (Mn2O7) in light of the fact that (a) fluorine is more electronegative than oxygen (b) fluorine doesn’t have d-orbitals (c) fluorine settles lower oxidation state (d) in covalent mixtures fluorine can frame single bond just while oxygen shapes twofold bond
Solution: (d) The most noteworthy oxidation condition of manganese in fluoride is +4 (MnF4) yet in oxides it is +7 (Mn2O7) in light of the fact that in covalent mixtures fluorine can frame single...
When fermented K2Cr2O7 arrangement is added to Sn2+ salts, Sn2+ changes to (a) Sn (b) Sn3+ (c) Sn4+ (d) Sn+
Solution: (c) When fermented K2Cr2O7 arrangement is added to Sn2+ salt, Sn2+ changes to Sn4+. The response is given underneath:
Which of the accompanying assertion isn’t right? (a) Copper frees hydrogen from acids (b) In its higher oxidation states, manganese structures stable mixtures with oxygen and fluorine (c) Mn3+ and Co3+ are oxidizing specialists in watery arrangement (d) Ti2+ and Cr2+ are decreasing specialists in watery arrangement
Solution: (a) Copper doesn't free hydrogen from acids. \[\begin{array}{*{35}{l}} Cu\text{ }+\text{ }2H2S04\text{ }\text{ }>\text{ }CuSO4\text{ }+\text{ }S02\text{ }+\text{ }2H2O \\ ~ \\...
KMn04 goes about as an oxidizing specialist in soluble medium. When soluble KMnO4 is treated with KI, iodide particle is oxidized to (a) I2 (b)Io–(c) I03 (d) I04
Solution: \[\left( c \right)\text{ }2KMnO4\text{ }+\text{ }KI\text{ }+\text{ }H2O\text{ }\text{ }>\text{ }2K0H\text{ }+\text{ }2MnO2\text{ }+\text{ }KI03\]
The attractive second is related with its twist rakish energy and orbital precise force. Twist just attractive second worth of Cr3+ particle is (a) 2.87 B.M. (b) 3.87 B.M. (c) 3.47 B.M. (d) 3.57 B.M.
Solution: (b) The attractive second is related with its twist precise force and orbital rakish energy.
Interstitial mixtures are shaped when little particles are caught inside the gem cross section of metals. Which of coming up next isn’t the trademark property of interstitial mixtures? (a) They have high liquefying focuses in contrast with unadulterated metals (b) They are exceptionally hard (c) They hold metallic conductivity (d) They are synthetically extremely receptive.
Solution: (d) Interstitial mixtures are synthetically idle.
Gadolinium has a place with 4f series. Its nuclear number is 64. Which of the following is the right electronic arrangement of gadolinium? (I) [Xe] 4f 75d16s2 (ii) [Xe] 4f 65d26s2 (iii) [Xe] 4f 86d2 (iv) [Xe] 4f 95s1
Solution: (a) 64Gd: [Xe] 4f7 5d1 6s2
Which of coming up next is amphoteric oxide? Mn2O7, CrO3, Cr2O3, CrO, V2O5, V2O4 (a) V2O5, Cr2O3 (b)Mn2O7, CrO3 (c) CrO, V2O5 (d) V2O5, V2O4
Solution: (a) V2O5 and Cr2O3 are amphoteric oxides on the grounds that both respond with alkalies just as acids. Keep in mind: In lower oxides, the fundamental person is transcendent while in higher...
KMnO4 goes about as an oxidizing specialist in acidic medium. The quantity of moles of KMnO4 that will be expected to respond with one mole of sulfide particles in acidic arrangement is (I) 2/5 (ii) 3/5 (iii) 4/5 (iv) 1/5
Solution:
There are 14 components in actinoid series. Which of the accompanying component doesn’t have a place with this series? (a) U (b) Np (c) Tm (d) Fm
Solution: (c) Tm (Thulium) is a lanthanoid.
At the point when KMnO4 arrangement is added to oxalic corrosive arrangement, the decolourisation is delayed to start with yet becomes momentary after some time on the grounds that (a) CO2 is shaped as the item (b) Reaction is exothermic (c) Mn04 catalyzes the response (d) Mn2+ goes about as autocatalyst
Solution: (d) When KMnO4 arrangement is added to oxalic corrosive arrangement, the decolourisation is delayed first and foremost yet becomes immediate after some time in light of the fact that Mn2+...
Which of the accompanying responses are disproportionation responses?
Solution:
Which of the accompanying oxidation state is normal for all lanthanoids? (a) +2 (b) +3 (c) +4 (d) +5
Solution: (b) +3 oxidation state is generally normal for all lanthanoids.
The attractive idea of components rely upon the presence of unpaired electrons. Distinguish the arrangement of change component, which shows the most elevated attractive second. (a) 3d7 (b) 3d5 (c) 3d8 (d) 3d2
Solution: (b) The more noteworthy the quantity of unpaired electron, the higher will be its worth of attractive second. Since, 3d5 has 5 unpaired electrons subsequently most noteworthy attractive...
On expansion of modest quantity of KMnO4 to concentrated H2SO4, a green slick compound is acquired which is exceptionally touchy in nature. Distinguish the compound from the accompanying: (a) Mn2O7 (b) MnO2 (c) MnSO4 (d) Mn2O3
Solution: \[\left( a \right)\text{ }2KMnO4\text{ }+\text{ }2H2SO4\left( Conc\text{ } \right)\text{ }\text{ }>\text{ }Mn2O7\text{ }+\text{ }2KHSO4\text{ }+\text{ }H2O\]
By and large, progress components structure shaded salts because of the presence of unpaired electrons. Which of the accompanying compound will be shaded in strong state? (a) Ag2SO4 (b) CUF2 (c) ZnF2 (d) Cu2Cl2
Solution: (b) Cu2+ has 1 unpaired electron in CuF2, consequently, it is hued in strong state.
Metallic radii of some change components are given underneath. Which of these components will have most noteworthy thickness?
Solution: (d) On moving passed on to directly along period, metallic span diminishes while mass increments. Diminishes in metallic span combined with expansion in nuclear mass outcomes in...
The electronic setup of Cu(II) is 3d9 while that of Cu(I) is 3d10. Which of coming up next is right? (I) Cu(II) is more steady (ii) Cu(II) is less steady (iii) Cu(I) and Cu(II) are similarly steady (iv) Stability of Cu(I) and Cu(II) relies upon the idea of copper salts
Solution: (a) Cu(II) is more steady because of more noteworthy compelling atomic charge of Cu(II).
Electronic design of a change component X in +3 oxidation state is [Ar]3d5. What is its nuclear number? (I) 25 (ii) 26 (iii) 27 (iv) 24
Solution:
Given the integers 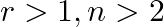 , and coefficients of
, and coefficients of 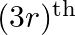 and
and 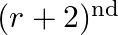 terms in the binomial expansion of
terms in the binomial expansion of 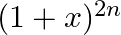 are equal, then
are equal, then
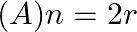
(B) 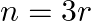
(C) 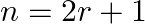
(D) none of these
(A) $n=2 r$ Explanation: Given expression is $(1+x)^{2 n}$ $T_{3 r}=T_{(3 r-1)+1}={ }^{2 n} C_{3 r-1} x^{3 r-1}$ $T_{r+2}=T_{(r+1)+1}={ }^{2 n} \mathrm{C}_{r+1} x^{r+1}$ ${ }^{2 n} C_{3 r-1}={ }^{2...
Find the term independent of 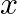 in the expansion of
in the expansion of 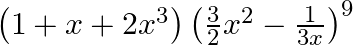
Given expression is, $\left(1+x+2 x^{3}\right)\left(\frac{3}{2} x^{2}-\frac{1}{3 x}\right)^{9}$ Considering $\left(\frac{3}{2} x^{2}-\frac{1}{3 x}\right)^{9}$ Using standard formula, we get...
If 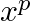 occurs in the expansion of
occurs in the expansion of 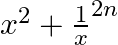 . Prove that its coefficient is
. Prove that its coefficient is 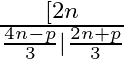
Given expression is $\left(x^{2}+\frac{1}{x}\right)^{2 n}$ Using the standard formula, we get, $T_{r+1}={ }^{2 n} C_{r}\left(x^{2}\right)^{2 n-r}\left(\frac{1}{x}\right)^{r}={ }^{2 n} C_{r} x^{4 n-3...
In the expansion of 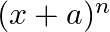 if the sum of odd terms is denoted by
if the sum of odd terms is denoted by  and the sum of even term by
and the sum of even term by  . Then prove that
. Then prove that
(i) 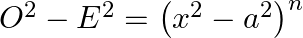
(ii) 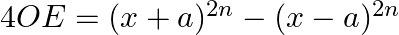
(i) We know, $(x+a)^{n}=$ ${ }^{n}C_{0} x^{n}+{ }^{n} C_{1} x^{n-1} a^{1}+{ }^{n} C_{2} x^{n-2} a^{2}+{ }^{n} C_{3} x^{n-3} a^{3}+\ldots$ Sum of odd terms will be, $O={ }^{n} C_{0} x^{n}+{ }^{n}...
The third term of G.P. is 4. The product of its first 5 terms is (A) 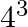 (B)
(B) 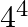 (C)
(C) 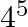 (D) None of these
(D) None of these
Solution: Option (C) 45 is correct. Explanation: It is given that the third term of G.P, ${{T}_{3}}~=\text{ }4$ We need to find the product of first 5 terms It is known that, ${{T}_{n}}~=\text{...
Show that the middle term in the expansion of 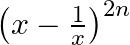 is
is 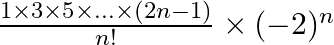
Given, expression is $\left(x-\frac{1}{x}\right)^{2 n}$. Since the index is given as $2 n$, which is even. So, there is only one middle term, i.e., $\left(\frac{2 n}{2}+1\right)$ th term $=(n+1)$ th...
If 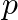 is a real number and if the middle term in the expansion of
is a real number and if the middle term in the expansion of 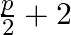 Is 1120 , find
Is 1120 , find 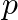 .
.
Given expansion is $\left(\frac{p}{2}+2\right)^{8}$ Since the index is given as $n=8$, there is only one middle term, i.e., $\left(\frac{8}{2}+1\right)$ th $=5^{\text {th }}$ term $T_{5}=T_{4+1}={...
Find the coefficient of 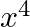 in the expansion of
in the expansion of 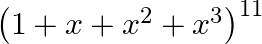
Given expression is $\left(1+x+x^{2}+x^{3}\right)^{11}$ $=\left[(1+x)+x^{2}(1+x)\right]^{11}=\left[(1+x)\left(1+x^{2}\right)\right]^{11}=(1+x)^{11} \cdot\left(1+x^{2}\right)^{11}$ $=\left({ }^{11}...
Find the value of 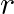 , if the coefficients of
, if the coefficients of 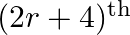 and
and 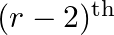 terms in the expansion of
terms in the expansion of 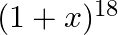 are equal.
are equal.
Given function is $(1+x)^{18}$ Now, $(2 r+4)^{\text {th }}$ term will be equal to $T_{(2 r+3)+1}$ $T_{(2 r+3)+1}={ }^{18} C_{2 r+3}(x)^{2 r+3}$ And $(r-2)^{\text {th }}$ term, that is $T_{(r-3)...
Find the coefficient of 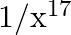 in the expansion of
in the expansion of 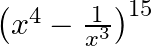
Given function is $\left(x^{4}-\frac{1}{x^{3}}\right)^{15}$ We may write the provided expression as $T_{r+1}$ using the conventional formula, $T_{r+1}={ }^{15}...
In a cricket tournament 16 school teams participated. A sum of Rs 8000 is to be awarded among themselves as prize money. If the last placed team is awarded Rs 275 in prize money and the award increases by the same amount for successive finishing places, how much amount will the first place team receive?
Solution: Let the sum got by the lead position group be a Rs and d be distinction in sum As the thing that matters is same subsequently the runner up will get a – d and the third spot a – 2d, etc...
In a potato race 20 potatoes are placed in a line at intervals of 4 metres with the first potato 24 metres from the starting point. A contestant is required to bring the potatoes back to the starting place one at a time. How far would he run in bringing back all the potatoes?
Solution: Given at start he needs to run 24m to get the main potato then 28 m as the following potato is 4m away from first, and so on Thus the succession of his running will be 24, 28, 32 … There...
Find the coefficient of 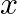 in the expansion of
in the expansion of 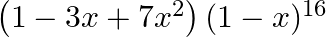
Given function is $\left(1-3 x+7 x^{2}\right)(1-x)^{16}$ Expansion of the function is, $=\left(1-3 x+7 x^{2}\right)\left({ }^{16} C_{0}-{ }^{16} C_{1} x^{1}+{ }^{16} C_{2} x^{2}+\ldots+{ }^{16}...
A side of an equilateral triangle is 20cm long. A second equilateral triangle is inscribed in it by joining the mid points of the sides of the first triangle. The process is continued as shown in the accompanying diagram. Find the perimeter of the sixth inscribed equilateral triangle.
Solution: Say ABC is a triangle with $AB\text{ }=\text{ }BC\text{ }=\text{ }AC\text{ }=\text{ }20$ cm Let’s say that D, E and F are respectively the midpoints of AC, CB and AB that are joined to...
We know the sum of the interior angles of a triangle is 180°. Show that the sums of the interior angles of polygons with 3, 4, 5, 6, … sides form an arithmetic progression. Find the sum of the interior angles for a 21-sided polygon.
Solution: It is given that the sum of interior angles of a polygon having ‘n’ sides is denoted by $(n-2)\times {{180}^{\circ }}$ The sum of angles having 3 sides i.e n $=\text{ }3\text{ }is\text{...
Find the term independent of 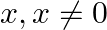 , in the expansion of
, in the expansion of 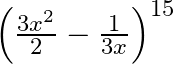
Given function is $\left(\frac{3 x^{2}}{2}-\frac{1}{3 x}\right)^{15}$ We know, the standard formula of $T_{r+1}$ will be, $T_{r+1}={ }^{15} C_{r}\left(\frac{3...
A carpenter was hired to build 192 window frames. The first day he made five frames and each day, thereafter he made two more frames than he made the day before. How many days did it take him to finish the job?
Solution: It is given that on the day first he made five frames then 2 frames more than the previous i.e. 7 and then 9 and so on Therefore the making of frames each day forms a sequence of 5, 7, 9…...
A man accepts a position with an initial salary of Rs 5200 per month. It is understood that he will receive an automatic increase of Rs 320 in the very next month and each month thereafter. (a) Find his salary for the tenth month (b) What is his total earnings during the first year?
Solution: It is given to us that in first month the man’s salary is Rs.5200 and then it increases by Rs.320 every month Therefore, 5200, 5200 + 320, 5200 + 640… will be the sequence so formed of his...
A man saved Rs 66000 in 20 years. In each succeeding year after the first year he saved Rs 200 more than what he saved in the previous year. How much did he save in the first year?
Solution: It is given that the total amount saved is 66000 in 20 years, therefore ${{S}_{20}}~=\text{ }66000$ Let ‘a’ be the amount saved in first year. Then every year if he increases Rs. 200 every...
Everybody in a room shakes hands with everybody else. The total number of hand shakes is 66. The total number of persons in the room is A. 11 B. 12 C. 13 D. 14
Solution: Option (B) 12 is correct. Explanation: It is known that, ${ }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}}$ $=\frac{n !}{r !(n-r) !}$ Let total time of handshakes $= {}^n{C}_2 = 66$...
Total number of words formed by 2 vowels and 3 consonants taken from 4 vowels and 5 consonants is equal to A. 60 B. 120 C. 7200 D. 720
Solution: Option (C) 7200 is correct. Explanation: It is known that, ${ }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}}$ $=\frac{n !}{r !(n-r) !}$ 4 be the total number of given vowel 5 be the total number...
A five digit number divisible by 3 is to be formed using the numbers 0, 1, 2, 3, 4 and 5 without repetitions. The total number of ways this can be done is A. 216 B. 600 C. 240 D. 3125 [Hint:5 digit numbers can be formed using digits 0, 1, 2, 4, 5 or by using digits 1, 2, 3, 4, 5 since sum of digits in these cases is divisible by 3.]
Solution: Option (A) 216 is correct Explanation: Using digits $0,1,2,4,5$ the 5-digit numbers that can be formed $$\begin{tabular}{|l|l|l|l|l|} \hline 4 & 4 & 3 & 2 & 1 \\ \hline \end{tabular}$$ $4...
The number of different four digit numbers that can be formed with the digits 2, 3, 4, 7 and using each digit only once is A. 120 B. 96 C. 24 D. 100
Solution: Option (C) 24 is correct. Explanation: It is known that, $\begin{array}{l} { }^{n} P_{r}= \\ \frac{n !}{(n-r) !} \end{array}$ ${ }^{4} \mathrm{P}_{4}=4 !=24$ is the four digit numbers that...
The number of possible outcomes when a coin is tossed 6 times is A. 36 B. 64 C. 12 D. 32
Solution: Option (B) 64 is correct. Explanation: The coin is tossed 6 times. 2 is the number of possible outcomes When a coin is tossed 6 times the number of possible outcomes is $2^ {6} = 64$ As a...
If 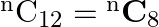 , then
, then 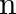 is equal to A. 20 B. 12 C. 6 D. 30
is equal to A. 20 B. 12 C. 6 D. 30
Solution: Option (A)20 is correct. Explanation: As per the question, ${ }^{\mathrm{n}} \mathrm{C}_{12}={ }^{\mathrm{n}} \mathrm{C}_{8}$ It is known that, $\begin{array}{l} { }^{\mathrm{n}}...
A group consists of 4 girls and 7 boys. In how many ways can a team of 5 members be selected if the team has (i) at least three girls.
Solution: It is known that ${ }^{n} C_{r}$ $=\frac{n !}{r !(n-r) !}$ (i) At least three girls The total no. of ways in which the team can have at least three girls $={ }^{4} \mathrm{C}_{3}{ }^{7}...
solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
A group consists of 4 girls and 7 boys. In how many ways can a team of 5 members be selected if the team has (i) no girls (ii) at least one boy and one girl
Solution: It is known that ${ }^{n} C_{r}$ $=\frac{n !}{r !(n-r) !}$ (i) No girls The total no. of ways the team can have no girls $={ }^{4} \mathrm{C}_{0}{ }^{7} \mathrm{C}_{5}=21$ (ii) at least...
solve the following:
14. Solution:
solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
Solve the following:
Solution:
A sports team of 11 students is to be constituted, choosing at least 5 from Class XI and atleast 5 from Class X II. If there are 20 students in each of these classes, in how many ways can the team be constituted?
Solution: It is known that, $=\frac{n !}{r^{n}} C_{r}$ In the following two ways a team of 11 students can be constituted (i) Five students from class $\mathrm{XI}$ and six students from...
Solve the following:
Solution:
solve the following:
Solution:
solve the following:
Solution:
In how many ways can a football team of 11 players be selected from 16 players? How many of them will (i) include 2 particular players? (ii) exclude 2 particular players?
Solution: It is known that, $=\frac{{ }^{n} C_{r}}{r !(n-r) !}$ As per the question, Out of 16, 11 players can be selected $={ }^{16} \mathrm{C}_{11}$ (i) include 2 particular players $={ }^{14}...
solve the following:
Solution:
solve the following:
Solution:
A bag contains six white marbles and five red marbles. Find the number of ways in which four marbles can be drawn from the bag if (a) they must all be of the same colour.
Solution: We know that, $\begin{array}{l} { }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}} \\ =\frac{n !}{r !(n-r) !} \end{array}$ As per the question, No. of white marbles is 6 No. of red marbles is 5...
Verify the following:
Solution:
Find the equation of each of the following parabolas (c) Focus at (–1, –2), directrix x – 2y + 3 = 0
Find the equation of each of the following parabolas (a) Directrix x = 0, focus at (6, 0) (b) Vertex at (0, 4), focus at (0, 2)
(a) The distance of any point on the parabola from its focus and its directrix is same. Given that, directrix, x = 0 and focus = (6, 0) If a parabola has a vertical axis, the standard form of the...
A bag contains six white marbles and five red marbles. Find the number of ways in which four marbles can be drawn from the bag if (a) they can be of any colour (b) two must be white and two red and
Solution: We know that, $\begin{array}{l} { }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}} \\ =\frac{n !}{r !(n-r) !} \end{array}$ As per the question, No. of white marbles is 6 No. of red marbles is 5...
Find the equation of a circle passing through the point (7, 3) having radius 3 units and whose centre lies on the line y = x – 1.
the equation of a circle having centre (h, k), having radius as r units, is (x – h)2 + (y – k)2 = r2Centre lies on the line i.e., y = x – 1, Co – Ordinates are (h, k) = (h, h – 1)...
Find the equation of a circle of radius 5 which is touching another circle x2 + y2 – 2x – 4y – 20 = 0 at (5, 5).
Given \[\begin{array}{*{35}{l}} {{x}^{2}}~\text{ }-2x\text{ }+\text{ }{{y}^{2}}~\text{ }-4y\text{ }-\text{ }20\text{ }=\text{ }0 \\ {{x}^{2}}~\text{ }-2x\text{ }+\text{ }1\text{ }+{{y}^{2}}~\text{...
Find the equation of a circle whose centre is (3, –1) and which cuts off a chord of length 6 units on the line 2x – 5y + 18 = 0.
Using Pythagoras Theorem, (Hypotenuse)2 = (Base)2 + (Perpendicular)2 = (3)2 + (√29)2 = 29 + 9 = √38 Hypotenuse = √38 units (radius) Since, the radius bisects the chord into two equal halves, Since,...
Find the equation of the circle which passes through the points (2, 3) and (4, 5) and the centre lies on the straight line y – 4x + 3 = 0.
the equation of a circle having centre (h, k), having radius as r units, is \[{{\left( x\text{ }-\text{ }h \right)}^{2}}~+\text{ }{{\left( y\text{ }-\text{ }k \right)}^{2}}~=\text{ }{{r}^{2}}\ldots...
If the lines 2x – 3y = 5 and 3x – 4y = 7 are the diameters of a circle of area 154square units, then obtain the equation of the circle.
Since, diameters of a circle intersect at the centre of a circle, \[\begin{array}{*{35}{l}} 2x\text{ }-\text{ }3y\text{ }=\text{ }5\text{ }\ldots \ldots \ldots 1 \\ 3x\text{ }-\text{ }4y\text{...
Find the equation of the hyperbola with eccentricity 3/2 and foci at (± 2, 0).
Find the eccentricity of the hyperbola 9y^2 – 4x^2 = 36.
If the distance between the foci of a hyperbola is 16 and its eccentricity is √2, then obtain the equation of the hyperbola.
If the line y = mx + 1 is tangent to the parabola y2 = 4x then find the value of m.
equations are, y = mx + 1 & y2 = 4x By solving given equations we get (mx + 1)2 = 4x Expanding the above equation we get \[{{m}^{2}}{{x}^{2}}~+\text{ }2mx\text{ }+\text{ }1\text{ }=\text{ }4x\]...
If the points (0, 4) and (0, 2) are respectively the vertex and focus of a parabola, then find the equation of the parabola.
Find the length of the line-segment joining the vertex of the parabola y2 = 4axand a point on the parabola where the line-segment makes an angle q to the x-axis.
A swimming pool is to be drained for cleaning. If L represents the number of litres of water in the pool t seconds after the pool has been plugged off to drain and L = 200 (10 – t)2. How fast is the water running out at the end of 5 seconds? What is the average rate at which the water flows out during the first 5 seconds?
Given, \[L\text{ }=\text{ }200\left( 10\text{ }\text{ }t \right)2\] where L addresses the quantity of liters of water in the pool. On separating both the sides w.r.t, t, we get \[dL/dt\text{...
Find the coordinates of a point on the parabola y2 = 8x whose focal distance is 4.
equation of an ellipse is y2 = 4ax, Also we have length of latus rectum = 4a Now by comparing the above two equations, 4a = 8 Therefore \[\begin{array}{*{35}{l}} a\text{ }=\text{ }2 \\...
