Answer: In a reaction between newly precipitated aluminum hydroxide and caustic soda solution, sodium meta aluminate is formed as a white salt. Al(OH)3 + NaOH → NaAlO2 {Sodium meta aluminate} +...
Select the correct answers:
(c) A metal which produces hydrogen on reacting with alkali as well as with acid.
(i) Iron (ii) Magnesium
(iii) Zinc (iv) Copper
(d) The salt solution which does not react with ammonium hydroxide is
(i) Calcium nitrate (ii) Zinc nitrate
(iii) Lead nitrate (iv) Copper nitrate
Solution: Copper also reacts with dil HCl and produces hydrogen and it also reacts with NaOH. (c) (iii) Zn + 2NaOH → Na2ZnO2 {Sodium zincate} (Colourless) + H2 Zn + HCl →...
What happens when Lead nitrate solution is mixed with dilute hydrochloric acid and heated.
Solution: When dilute HCl is added to the lead nitrate (Pb(NO3)2) solution and heated, it forms a white precipitate of lead chloride (PbCl2) and nitric acid. A double displacement reaction occurs in...
Write the balanced equations for the following reactions: (i)Iron + dilute hydrochloric acid (ii)Ammonia + dilute hydrochloric acid (iii)Iron(II) sulphide +dilute hydrochloric acid (iv)Magnesium sulphite + dilute hydrochloric acid (v)Sodium hydrogen carbonate + dilute hydrochloric acid
Solution: Fe + 2HCl → FeCl2 + H2 NH3 + HCl →NH4Cl FeS + 2HCl →FeCl2 + H2S MgSO3 + 2HCl → MgCl2 + H2O + SO2 NaHCO3 + HCl → NaCl + H2O + CO2
Select the correct answers:
(a) Colour of an aqueous solution of copper sulphate is
(i) Green (ii) Brown
(iii) Blue (iv) Yellow
(b) Colour of the precipitate formed on adding NaOH solution to iron (II) sulphate solution is
(i) White (ii) Brown
(iii) Green (iv) Pale blue
Solution: (a) (iii) An aqueous solution of copper sulfate is blue. (b) (iii) FeSO4 {Dirty green} + 2NaOH → Fe(OH)2 ↓ {gelatinous ppt.} + Na2SO4
Name
(a) A yellow monoxide that dissolves in hot and concentrated caustic alkali.
(b) A white, insoluble oxide that dissolves when fused with caustic soda or caustic potash.
(c) A compound containing zinc in the anion.
Answer: (a) PbO- Lead Oxide (b) ZnO- Zinc Oxide (c) K2ZnO2
On adding dilute ammonia solution to the colorless solution of a salt, a white gelatinous precipitate appears. This precipitate, however, dissolves in the addition of an excess of ammonia solution. Identity (choose from Na, Al, Zn,Pb, Fe)
(a) Which metal salt solution was used?
(b) What is the formula of the white gelatinous precipitate obtained?
Solution: (a) Zinc metal salt solution was used. $ZnCl_2$(b) The formula of white gelatinous precipitate is $Zn(OH)_2$
Complete the following equation: (i)Silver nitrate solution + hydrochloric acid → (ii)Magnesium foil + hydrochloric acid → (iii)Caustic soda solution + hydrochloric acid → (iv)Zinc carbonate + hydrochloric acid → (v)Copper oxide + hydrochloric acid →
Solution: AgNO3 + HCl → AgCl ↓+ HNO3 Mg + HCl → MgCl2 + H2 ↑ NaOH + HCl → NaCl + H2O ZnCO3 + 2HCl → ZnCl2 + H2O + CO2 ↑ CuO + 2HCl → CuCl2 + H2O
A solution of hydrogen chloride in water is prepared. The following substances are added to separate portions of the solution. Complete the table by writing the gas evolved in each case and its odour.
Substance Added Gas Evolved Odour Calcium Carbonate Magnesium ribbon Manganese(IV) oxide with heating Sodium sulphide Solution:
How to convert Hydrochloric acid to nascent chlorine? Explain with a balanced equation.
Solution: Three parts concentrated hydrochloric acid and one part concentrated nitric acid can be used together to produce nascent chlorine. 3HCl (conc) + HNO3(conc) → NOCl + 2H2O + 2...
What are the uses of Hydrochloric Acid?
Solution: The following are some of the applications of hydrochloric acid: Table salt is purified using this method. They're used as pH regulators and neutralizers. To clean pools, it's used as a...
What are the physical properties of Hydrogen Chloride Gas?
Solution: Hydrogen Chloride (HCl)Gas Physical Properties It's a colourless gas.It has a sour flavour and a strong odour.It is readily soluble in non-polar solvents such as water.The melting point of...
Name the chloride of a metal that is soluble in excess of ammonium hydroxide. Write an equation for the same.
Answer: Zinc chloride (ZnCl2) is soluble in excess of ammonium hydroxide. ZnCl2 + 2NH4OH → Zn(OH)2 ↓ {White gelatinous ppt.} + 2NH4Cl With an excess of NH4OH ppt. dissolves Zn(OH)2 + 2NH4Cl +...
What do you observe when caustic soda solution is added to the following solution: first a little and then in excess.
(c) 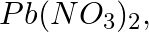
(d) 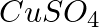 ? Write balanced equations for these reactions.
? Write balanced equations for these reactions.
Solution: Caustic soda is Sodium hydroxide (NaOH) (c) Pb(NO3)2 + 2NaOH → Pb(OH)2 ↓ {White ppt. (colourless)}+ 2NaNO3 In excess of alkali, the white precipitate of Pb(OH)2 becomes soluble. Pb(OH)2 +...
What do you observe when caustic soda solution is added to the following solution: first a little and then in excess.
(a) 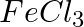 , (b)
, (b) 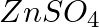 Write balanced equations for these reactions.
Write balanced equations for these reactions.
Solution: Caustic soda is Sodium hydroxide (NaOH) (a) FeCl3 + 3NaOH → Fe(OH)3 ↓ {Reddish-brown ppt.}+ 3NaCl In excess of alkali, the reddish-brown ppt. of Fe(OH)3 remains insoluble. (b) ZnSO4 +...
What happens when ammonia solution is added first dropwise and then in excess to the following solutions: (i) CuSO4 (ii) ZnSO4 (iii)FeCl3. Write balanced equations for these reactions.
Solution: (i) CuSO4 + 2NH4OH → Cu(OH)2 ↓ + (NH4)2SO4 With an excess of NH4OH, ppt. dissolves Cu(OH)2 + (NH4)2SO4 + 2NH4OH → [Cu(NH3)4]SO4 + 4H2O ii) ZnSO4 + 2NH4OH → Zn(OH)2 ↓ + (NH4)2SO4 With an...
What happens when ammonia solution is added first dropwise and then in excess to the following solutions: (iii)FeCl3. Write balanced equations for these reactions.
Answer: FeCl3 + 3NH4OH → Fe(OH)3 ↓ + 3NH4Cl
Complete and balance the following reactions: (i)NH4OH + HCl→ (ii)Pb(NO3)2 +HCl→ (iii)HCL + NH4OH→ (iv)NH3 + HCL→
Solution: NH4OH + HCl →NH4Cl + H2O. Pb(NO3)2 +HCl →PbCl2 +2HNO3. HCl + NH4OH → NH4Cl + H2O. NH3 + HCl → NH4Cl.
How will you prove that hydrochloric acid contains hydrogen chlorine?
Solution: The following experiment can be used to demonstrate that hydrochloric acid contains both hydrogen and chlorine.Step 1: Prepare a voltameter for water electrolysis with a platinum cathode...
Write balanced equations for Q.2 (g) and (i).
Solution: For 2(g): For 2(i): PbO + 2NaOH → Na2PbO2 + H2O (Yellow) Sodium Plumbate (Colourless, soluble)
Name
(i) a colored metallic oxide that dissolves in alkalis to yield colorless solutions.
(j) a colourless cation, not a representative element.
Answer: (i) PbO (j) Ammonium ion
Name
(g) a metal that evolves into a gas that burns with a pop sound when boiled with alkali solutions.
(h) Two bases that are not alkalis but dissolve in strong alkalis.
Answer: (g) Aluminium (h) Zn(OH)2 and Al(OH)3
Give examples of two colourless gases, which combine to produce a white solid.
Solution: Hydrogen chloride and ammonia react to form ammonium chloride.
Name:
(e) Two colourless metal ions.
(f) Two coloured metal ions.
Answer: (e) Na+, Ca2+ (f) Fe2+, Mn2+
Name:
(c) a strong alkali.
(d) a weak alkali.
Answer: (c) NaOH : A strong alkali dissociates completely to form OH- ions. (d) NH4OH: A weak alkali only partially dissociates to form OH- ions.
Name:
(a) a metallic hydroxide soluble in excess of 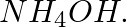
(b) a metallic oxide soluble in excess of caustic soda solution.
Answer: (a) Cu(OH)2 : Copper (II) hydroxide is amphoteric in nature and soluble in excess ammonium hydroxide. (b) ZnO: It is amphoteric which means that it dissolves in both acidic and basic...
The dilute hydrochloric acid cannot be concentrated by boiling beyond 22.2 per cent. Why?
Solution: Because the molecules of dilute hydrochloric acid HCl(g) get mixed with water vapour. A solution of HCl gas in water is known as dilute HCl. (i) The greatest concentration achieved at 20°C...
Which acid is used in the preparations of Hydrogen Chloride gas?
Solution: Concentrated Sulfuric acid (H2SO4) (i) Sodium chloride is treated with strong sulphuric acid to produce hydrogen chloride gas. To remove moisture, the HCl gas is delivered through a drying...
Write the probable color of the following salts?
(a) Ferrous salts (b) Ammonium salts
(c) Cupric salts (d) Calcium salts
(e) Aluminium salts
Answer: (a) Ferrous salts – Light green (b) Ammonium salts – Colourless (c) Cupric salts – Blue (d) Calcium salts – Colourless (e) Aluminium salts...
What is STP?
Answer: When it comes to experimental measurements, standard temperature and pressure are standard sets of circumstances that must be created in order for comparisons to be made between different...
State two real-life examples of Avogadro’s Law.
Answer: It is described by Avogadro's law, which describes the link between the volume of a gas and the number of molecules contained inside it. It was first proposed in 1811 by an Italian physicist...
Mention some applications of Avogadro’s Law
Answer: The law describes Gay-law Lussac's of combining volumes, which is a mathematical formula.It is responsible for determining the atomicity of gases.Contributes to determining a gas's molecular...
What is relative atomic mass?
Answer The ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant is a dimensionless physical quantity defined as the ratio of the average mass of...
State the value of Avogadro number
Answer: Avogadro's number is equal to $6.02214076$ $\times $ $10^{23}$, which is the number of units contained in one mole of any material (defined as its molecular weight in grams).
What is the atomicity of phosphorous, sulphur and hydrogen?
Answer: Atomicity is defined as the total number of atoms present in a molecule. Atomicity is measured in parts per million. For example, the molecule of oxygen is made up of two oxygen atoms per...
What is atomicity?
Atomicity is defined as the total number of atoms present in a molecule. Atomicity is measured in parts per million. For example, the molecule of oxygen is made up of two oxygen atoms per molecule....
What is meant by an empirical formula of a compound?
Answer: According to chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of all of the atoms that are present in the complex. As a basic illustration of...
What is stoichiometry?
Answer: In chemistry, the term "stoichiometry" refers to the relationship between the quantities of reactants and products present before, during, and after chemical processes. Stoichiometry is...
State Gay-Lussac’s law of combining volumes
Answer: Gay-law Lussac's of combining volumes asserts that, when distinct gases react together, they do so in terms of volume that bears a simple whole-number ratio, given that the temperature and...
State Gay-Lussac’s law
Answer: When the volume of a gas is held constant, Gay-Law Lussac asserts that the pressure of a given amount of gas varies directly with the absolute temperature of the gas. During the cooling...
Calculate the volume of 7.1 kg of Cl at Standard temperature and pressure.
Solution: Calculating the volume: Gram molecular weight of $Cl_{2}$ (one mole) $=35.5 \times 2=71 g$. $71 g$ of $Cl {2}$ at S.T.P occupies $22.4$ litres.$\therefore 7.1 kg$, i.e., $7100 g$ of $Cl...
The atomic weight of Cl is 35.5. Calculate its vapor density.
Solution:The vapor density is defined as the mass of n molecules of gas divided by the mass of n molecules of hydrogen.The vapor density is equal to the molar mass of gas divided by the molar mass...
What is the volume of propane burnt for every 0.1 litres of oxygen in the following reaction? Note: volumes of gases are measured at room pressure and temperature. 
Solution: When the volume of a gas is held constant, Gay-law Lussac asserts that the pressure of a given amount of gas varies directly with the absolute temperature of the gas, and vice versa. From...
Explain: “The vapour density of carbon dioxide is 22.”
Answer: The weight of a certain molecule of gas (in this case, carbon dioxide) is the same as the weight of a molecule of hydrogen (22), provided that both gases are at the same temperature and...
Explain: “The number of atoms in 1 mole of hydrogen is twice the number of atoms in 1 mole of helium at the same pressure and temperature.”
Answer: The reason for this is that hydrogen gas is diatomic, but helium gas is monoatomic, and as a result, they are diatomic. When comparing the number of atoms in one mole of hydrogen to the...
What is the meaning of “Molar volume of a gas?”
Answer: One mole of a material (gas) occupies the volume equal to the volume filled by one mole of the substance (gas) at normal temperature and pressure. The experimental value gas at STP is 22.4...
State Avogadro’s law.
Answer: It is stated that "equivalent volumes of all gases at the same temperature and pressure have the same number of molecules," according to Avogadro's Law. If the temperature and pressure of an...
One word answers:
1. Average of the ratio of atoms present in an element
2. The mass (in grams) of one mole of a molecular compound
3. A formula that tells us the number of atoms in one molecule of a chemical substance
Solution: Atomic weightGram-molecular weightMolecular formula Explanation: 1. The atomic mass is the mass of a single atom in the universe. In spite of the fact that the kilogramme is the standard...
Fill in the blanks:
5. The number of particles that are contained within one mole of a substance is called _________
Solution: One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro's number or Avogadro's constant....
Fill in the blanks:
3. ____________ (molecule/atom) is classified as the smallest unit of matter.
4. Equal volumes of all ____________ (gases/liquids), under similar conditions of pressure and temperature contains an equal number of molecules.’
Solution: 3. Atom This is a fact the atom is the smallest unit of matter. 4. Gases When equal volumes of different gases are mixed under the same conditions of temperature and pressure, Avogadro's...
Fill in the blanks: 1. Relative molecular mass is essentially a number that tells us how many times a single _________(molecule/atom) of a substance is heavier than 1/12th mass of carbon [12/6C].
2. Whenever gases chemically react, they occur in ______________ (volume/weight) which bear a simple ratio to the products and each other. If gaseous, provided the pressure and temperature of reacting gases and products remain the same.
Solution: Answer: 1. Molecule When using a relative scale, the mass of 12C (carbon twelve) is defined as 12 amu; therefore, this is an accurate number. Atomic mass is measured in amu. They tell us...
(a) In Period 3 of the Periodic table, element B is placed to the left of element A. On the basis of this information, choose the correct word from the brackets to complete the following statements:
(i) The element B would have (lower /higher) metallic character than A. (ii) The element A would probably have (lesser / higher) electron affinity than B. (iii) The element A would have...
(a) Metals are good _____________ (oxidising agents/reducing agents) because they are electron __________ (acceptors/donors).
(b) An element with the atomic number 19 will most likely combine chemically with the element whose atomic number is: (i) 17 (ii) 11 (iii) 18 (iv) 20 (c) Rewrite the...
(a) Among the elements given below, the element with the least electronegativity is
(i) Lithium (ii) Carbon (iii) Boron (iv) Fluorine (b) The metals of Group 2 from top to bottom are Be, Mg, Ca, Sr and Ba. (i) Which of these elements will form ions most...
(a) Choose the correct answer from the choice given:
(i) Ionisation potential increases over a period from left to right because the A. Atomic radius and nuclear charge increase B. Atomic radius and nuclear charge decrease C. Atomic radius...
(a) Among the Period 2 elements, the element which has high electron affinity is
A. Lithium B. Carbon C. Chlorine D. Fluorine (b) Group No.1-1A2-IIA13-IIIA14-IVA15-VA16-VIA17-VIIA18-02nd periodLiDOJNe3rd periodAMgESiHM4th periodRTIQUY In the above...
(a) Choose the correct answer from the option:
An element in Period 3 whose electronic affinity is zero. A. Neon B. Sulphur C. Sodium D. Argon (b) Give reason: (i) The ionisation potential of the element increases across a period. (ii)...
(a) Give reasons – The oxidising power of elements increases from left to right along a period.
(b) Select the correct answer: (i) Across a period, the ionisation potential ………… [increases, decreases, remains the same] (ii) Down the group, electron affinity ………… [increases, decreases, remains...
(a) The number of electrons in the valence shell of a halogen is ………. A – 1, B – 3, C – 5, D – 7.
(b) Electronegativity across the period ………… [increases/decreases]. (c) Non-metallic character down the group ………… [increases/decreases]. (d) Atomic number of an element is 16. State (i) to which...
(a) Among Period 2 elements A, B, C and D, the one which has high electron affinity is
A. Lithium B. Carbon C. Fluorine D. Neon (b) Group No. ’s IAIIAIIIAIVAVAVIAVIIA012131415161718LiDOJNeAMgESiHKBCFGL Select from the table: (i) Which is the most electronegative? (ii) How...
Select the correct answer from the choices A, B, C, D which are given. Write down only the letter corresponding to correct answer. With reference to the variation of properties in the Periodic table, which of the following is generally true?
(a) Atomic size increases from left to right across a period. (b) Ionization potential increases from left to right across a period. (c) Electron affinity increases going down a group. (d)...
The elements of one short period of the periodic table are given below in order from left to right:
Li, Be, B, C, O, F, Ne (a) To which period do these elements belong? (b) One element of this period is missing. Which is the missing element and where should it be placed? (c) Place the three...
Parts (a) to (e) refer to change in the properties of elements on moving from left to right across a period of the periodic table. For each property, choose the correct answer.
(a) The non-metallic character of the elements: (i) Decreases (ii) Increases (iii) Remains the same (iv) Depends on the period (b) The electronegativity: (i) Depends on the number of valence...
Choose the word or phrase from the brackets which correctly completes each of the following statements:
(a) The element below sodium in the same group would be expected to have a …………… (lower/higher) electro-negativity than sodium, and the element above chlorine would be expected to have a...
The electronegativities (according to Pauling) of the elements in Period 3 of the Periodic Table are as follows with elements arranged in alphabetical order:
AlClMgNaPSSi1.531.20.92.12.51.8 Arrange the elements in the order in which they occur in the periodic table from left to right. (The group 1 element first, followed by the group 2 element and so on,...
Which element from the following has the highest ionization energy?
(a) P, Na, Cl (b) F, O, Ne (c) Ne, He, Ar Explain your choice. Answers: (a) Cl has the highest ionization energy. Normally, non-metals have high ionisation energy when...
Arrange the following in order of increasing radii:
(a) Cl¯, Cl (b) Mg2+, Mg, Mg+ (c) N, O, P Answers: (a) Cl < Cl¯ (in order of increasing radii) (b) Mg2+ < Mg+ < Mg (in order of increasing radii) (c) O...
First Ionization enthalpy of two elements X and Y are 500 kJ/mol-1 and 375 kJ /mol -1 respectively. Comment about their relative position in a group as well in a period.
The ionization enthalpy of an element is the minimum amount of energy needed to remove the outermost electron from a gaseous neutral atom. Given, The relative position in a group: X is above the Y...
Choose the most appropriate answer from
[SO2, SiO2, Al2O3, CO, MgO, Na2O] (a) A covalent oxide of a metalloid. (b) An oxide which when dissolved in water forms acid. (c) A basic oxide. (d) An amphoteric oxide. ...
Chorine in the Periodic Table is surrounded by the elements with atomic number 9, 16, 18 and 35.
(a) Which of these have physical and chemical properties resembling chlorine? (b) Which is more electronegative than chlorine? Answers: (a) The elements having the atomic number of 9 and 35 have...
Arrange the following as per instructions given in the brackets.
(a) Mg, Cl, Na, S, Si (increasing order of atomic size) (b) Cs, Na, Li, K, Rb (increasing metallic character) (c) Na, K, Cl, S, Si (increasing ionisation potential) (d) Cl, F, Br, I...
Name
(a) An alkali metal in period 3 and halogen in period 2 (b) The noble gas with 3 shells (c) The non-metals present in period 2 and metals in period 3. (d) The element of period 3...
Choose the correct option: (a) The metal other than aluminum, which has a strong affinity for oxygen is:
(A) Copper
(B) Magnesium
(C) Silver
(D) Gold
(b) A metallic oxide which cannot be reduced by normal reducing agents:
(A) Zinc oxide
(B) Magnesium oxide
(C) Copper (II) oxide
(D) Iron (III) oxide
Answer: (a) The answer is (B) Magnesium because magnesium can easily react with oxygen to undergo oxidation reaction forming magnesium oxide (b) The answer is (B) Magnesium oxide. Magnesium...
Fill in the blanks:(a) Usually __________ (sulphide/carbonate) ores are subjected to _________ (calcination/roasting) which is done in the absence of air
(b) Zinc blende is converted to oxide by ___________ (roasting/calcination) process.
(c) Froth floatation process is generally used to concentrate __________ ores (sulphides/carbonate)
Answer: (a) Carbonate, calcination (b) Roasting (c) Sulfides
State the position of aluminum in the periodic table.
Aluminum, the most prevalent metal in the earth's crust, belongs to Period 3, Group IIIA of the periodic table (13)
Name:
(a) the process involved in (i) dressing of the ores (ii) refining of the ores
(b) two metallic oxides which cannot be reduced by carbon, carbon monoxide, or hydrogen
Answer: (a) The processes involved in: (i) Dressing of ores are: Hydraulic washing Magnetic separation Froth floatation Chemical method/Leaching (ii) Refining of ores are:...
(a) Give the chemical names and formulae of main ores of (i) aluminum (ii) iron (iii) zinc
(b) Which impurities are present in bauxite?
(c) What is red mud, how is it removed?
(a) The chemical names and formulae of main ores have been mentioned below: (b) Aluminum oxide makes up to 60% of the bauxite ore. Ferric oxide, sand, and titanium oxide are the other...
For each substance listed below, explain its significance in the extraction of aluminium:
(a) bauxite
(b) sodium hydroxide
The significance of bauxite and sodium hydroxide in the extraction of aluminium had been mentioned below:
For each substance listed below, explain its significance in the extraction of aluminium:
(a) Cryolite (b) Graphite Name of the substanceImportance in aluminium extraction(a) Cryolite It is utilized to lower fusion temperatures while also increasing conductivity.(b) Graphite Thick...
What is an alloy? How do the properties of an alloy differ from its constituents?
An alloy is a homogenous mixture of two or more metals or one or more metals with a few non-metallic elements Qualities of alloy differ from those of its constituents. Gold, for example, is far too...
What is amalgam? State its use with an example.
An amalgam is a mixture or alloy of mercury with a variety of metals or a mixture of silver, gold, zinc, sodium, and non-metals. An example of amalgam is a dental amalgam made up of mercury and a...
Choose the correct option:
(a) The metal other than aluminum, which has a strong affinity for oxygen is:
(A) Copper (B) Magnesium
(C) Silver (D) Gold
(b) A metallic oxide which cannot be reduced by normal reducing agents:
(A) Zinc oxide (B) Magnesium oxide
(C) Copper (II) oxide (D) Iron (III) oxide
Answer: (a) The answer is (B) Magnesium. (b) The answer is (A) Zinc oxide
Define calcination. Give an example and equation for calcination.
It is the process of heating concentrated ores like carbonate or hydrated oxide to a high temperature in the absence of air. The mineral must be too hard to melt at this temperature. Example - Metal...
Define roasting. Name an ore on which roasting is done. Give a balanced equation.
Roasting is a technique that involves heating concentrated ore to a high temperature in the presence of air. Zinc blende (ZnS) is an ore that undergoes roasting to produce its oxide, zinc oxide. The...
An ore on being heated in air forms sulfurous anhydride. Write the process used for the concentration of this ore.
The froth flotation method was employed to concentrate this ore. This technique is based on the gangue particles' preference for water wettability over the ore's preference for pine oil wettability.
(a)Name an ore of zinc
(b) Which process is applied to concentrate it?
(c) How is concentrated ore changed to oxide?
Answer: (a) Zinc blende is a type of zinc ore. Zinc sulfide is its chemical name, and its formula is ZnS. (b) The method used to concentrate it is the froth flotation method. (c) By heating...
What do you observe when hydrogen is passed over heated copper oxide?
When hydrogen gas is passed over heated copper oxide (CuO), a black coating present on the surface give reddish-brown colour due to reverse reaction. Consequently, Copper is produced. The redox...
Why does iron or zinc does not occur freely in nature?
Because iron and zinc are very reactive, they do not exist in their free states in nature. Their metal compounds — sulfides, oxides, and carbonates – are found in nature.
(a) Name the methods by which concentrated ore is converted to metallic oxide
(b) State three objectives achieved during the roasting of ores.
Answer: (a) Calcination and roasting are two processes for converting concentrated ore into metallic oxides. Because oxides are easier to reduce to metals, concentrated ores are transformed...
Give the principle of the electromagnetic separation method of metals.
The magnetic characteristics of metal particles and gauge particles are used in this procedure. On the roller belt, which is a magnetic belt, powdered ore is inserted. As a result, the magnetic...
Give the principles of (a) Hydrolytic method (b) Froth floatation process
(a) Hydrolytic method - The fundamental criterion is the difference in ore and gangue densities. We pour the ore over a sloping, vibrating corrugated table with grooves in this approach. Water is...
Which metal can be extracted from haematite?
Haematite ore is used to extract iron from it. Iron is extracted from iron ore in a huge container called a blast furnace. Haematite contains iron (III) oxide, Fe2O3. The oxygen must be removed from...
Which metal can be extracted from each one of the following ores:
(a) bauxite
(b) calamine
Answer: The metals that can be extracted from each one of the following ores are as follows: (a) Bauxite – Aluminum (b) Calamine – Zinc
Define the term Metallurgy.
Metallurgy is the branch of science concerned with extracting metals in their purest form from the ores that exist in nature.
Explain the following terms: (a) ore (b) Gangue
(a) Ore - These are the minerals from which metals are commercially mined at a low cost and with minimal effort. (b) Gangue - The earthy impurities that are frequently combined with metal minerals...
Why do gold ornaments look new even after several years of use?
Because gold is less reactive, ornaments made of gold appear new even after several years of use. This may be seen in the reactivity series, where it is positioned at the bottom. As a result, when...
State the position of the following in the periodic table:
(a) Halogens
(b) Aluminum
Answer: (a) Halogens are the elements in Group 17 of the periodic table. Their electronic configuration is ns2 np5. (b) Aluminum is a member of the Boron family. It belongs to the 13th group...
State the position of the following in the periodic table:
(a) Alkali metals
(b) Alkaline earth metals
Answer: The position of the following in the periodic table are: (a) Alkali metals: These are elements in the periodic table that belong to Group I. They are known for forming strong alkalis...
Name the metal which is a constituent of:
(a) blood pigment
(b) plant pigment
The metals listed below are a component of the following: (a) Blood pigment – iron is a component of hemoglobin (b) Plant pigment – magnesium is found in chlorophyll
Answer the following questions:
a)Name the three classes in which elements are classified. (b) Which was the first metal used by man? Answer: (a) Metals, non-metals, and metalloids are the three types of elements classified....
