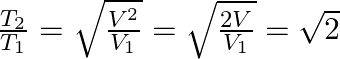a) find the work done when the gas is taken from state 1 to state 2
b) what is the ratio of temperature T1/T2 if V2 = 2V1
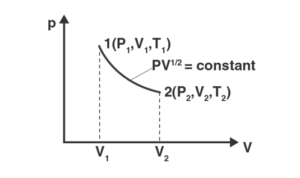
Answer:
According to the question, PV1/2 = K = constant
a) Expression for the work done for the process 1 to 2 is:
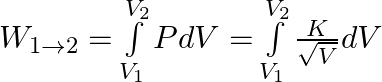
Solving the above equation, we get
![]()
b) We know that the ideal gas equation is
PV = nRT
Or, T = PV/nR
![]()
![]()