Deuterium was discovered in 1932 by Harold Urey by measuring the small change in wavelength for a particular transition in
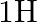
and
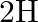
. This is because the wavelength of transition depends to a certain extent on the nuclear mass. If nuclear motion is taken into account then the electrons and nucleus revolve around their common centre of mass. Such a system is equivalent to a single particle with a reduced mass
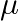
, revolving around the nucleus at a distance equal to the electron-nucleus separation. Here
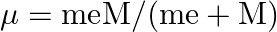
where
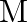
is the nuclear mass and
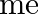
is the electronic mass. Estimate the percentage difference in wavelength for the 1 st line of the Lyman series in
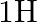
and
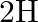
. (Mass of
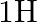
nucleus is
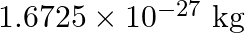
, Mass of
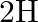
nucleus is
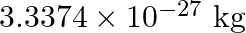
, Mass of electron
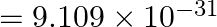
kg.)
![]()
![]()
![]()
![]()
![]()
![]()
