Have you ever wondered what are amphoteric oxides? Chances are you haven’t, but if you’re a chemist or a science enthusiast, it’s something you should definitely know.
In this article, we will discuss what amphoteric oxides are, how they form, and their applications in different industries.
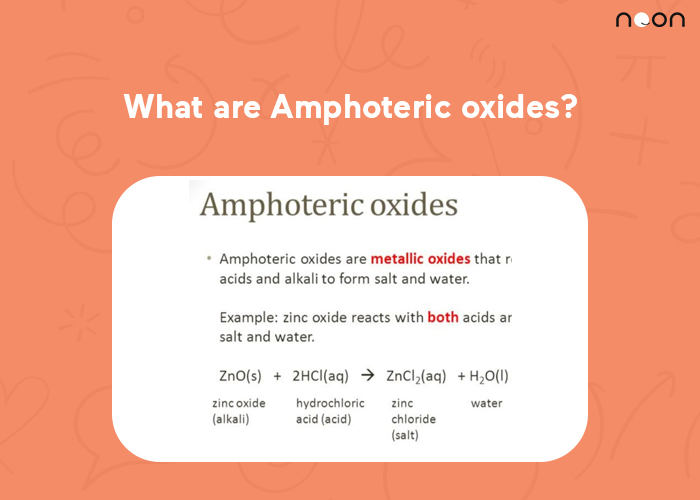
What are Amphoteric oxides?
An amphoteric oxide is a compound that can act as either an acid or a base. The term “amphoteric” comes from the Greek words “Amphi,” meaning “both,” and “teros,” meaning “to turn.” Amphoteric oxides are able to turn because they have both acidic and basic properties.
Are amphoteric oxides soluble in water? The answer is Yes, they dissolve in water to form an alkaline solution.
Amphoteric oxides are important in many industrial processes, such as the production of paper, glass, and ceramic products. They are also used in the manufacture of metals and alloys.
Amphoteric oxides Examples
There are many amphoteric oxides, but some of the most common are aluminum oxide, zinc oxide, and tin oxide. These materials have a Lewis acid-base reaction with water to form hydroxides.
Aluminum oxide is a white powder that is used as an abrasive and as a catalyst. It is also used in the manufacture of glass and ceramics.
Zinc oxide is a white powder that is used as an additive in plastics, rubbers, and paints. It is also used in the manufacturing of semiconductors.
Tin oxide is a white powder that is used as an additive in glass, ceramic, and paint production. It can also be used as a catalyst.
The different types of Amphoteric oxides
There are three different types of amphoteric oxides: neutral oxides, acidic oxides, and basic oxides.
- Neutral oxides are those that react with both acids and bases to form salt and water. Examples of neutral oxides include aluminum oxide and zinc oxide.
- Acidic oxides are those that react with bases to form salt and water, but not with acids. Examples of acidic oxides include tin oxide and lead oxide.
- Basic oxides are those that react with acids to form salt and water, but not with bases. Examples of basic oxides include magnesium oxide and calcium oxide.
The Properties of Amphoteric oxides
When amphoteric oxides are dissolved in water, they can either donate or accept protons (H+ ions). This makes them either acidic or basic, depending on the solution’s pH. For example, aluminum oxide is amphoteric and will act as an acid in a basic solution and a base in an acidic solution.
Amphoteric oxides can be used as buffers to maintain a particular pH in a solution. This is because they can neutralize both acids and bases.
Buffers are important in many chemical processes and are used to keep the pH of solutions within a certain range.
Conclusion
In conclusion, amphoteric oxides are compounds that can act as both acids and bases in different environments. They have a wide range of applications in industry, such as being used to help create detergents and other cleaning agents, or for use in the production of fertilizers.
Understanding how these compounds work is essential for anyone interested in chemistry to learn about, so if you want to become a chemist it is important that you understand them.
Noon is an application for online learning with professional teachers. It has over 10,000 lectures on different subjects from the best teachers from across the globe.
You can use the Noon app to learn more about other subjects and ace your next exam.
The ultimate teacher’s assistant tool for e-learning, Noon provides teachers with tools for online teaching, including video streams online and high-quality videos with interactive classrooms.
Thanks for reading this article on what amphoteric oxides are, now, download the Noon app to learn more about other types of Oxides