Acids and bases are two of the most fundamental concepts in chemistry. While acids and bases may seem like opposites, they’re actually closely related.
Acids and bases can interact in a number of ways and can even neutralize each other when combined. But, before you dive into these reactions, it’s important to understand the basics.
In this blog post, we will discuss what acids and bases are, what is the acidity test, and how to differentiate between them. So, read on to learn more about acids and bases!
What is an Acid?
An acid is a substance that donates protons (H+ ions) to another substance. In aqueous solutions, acids increase the concentration of H+ ions. The strength of an acid is measured by its ability to donate protons. Strong acids completely dissociate into H+ and an anion in water. Weak acids only partially dissociate into H+ and an anion in water.
What is an Acidity test?
An acidity test is a test used to determine the acidity or basicity of a solution. There are many different types of acidity tests, but they all work by measuring the hydrogen ion concentration in a solution. The most common type of acidity test is the pH test, which uses a pH meter to measure the hydrogen ion concentration in a solution.
What is a Base?
Bases are substances that increase the hydroxide ion (OH-) concentration in water. The most common base is sodium hydroxide (NaOH), which is also called lye.
Bases can be either strong or weak. Strong bases completely dissociate in water, while weak bases only partially dissociate.
Bases are often used to neutralize acids. For example, if you spill acid on your skin, you would neutralize it with a base such as baking soda (sodium bicarbonate).
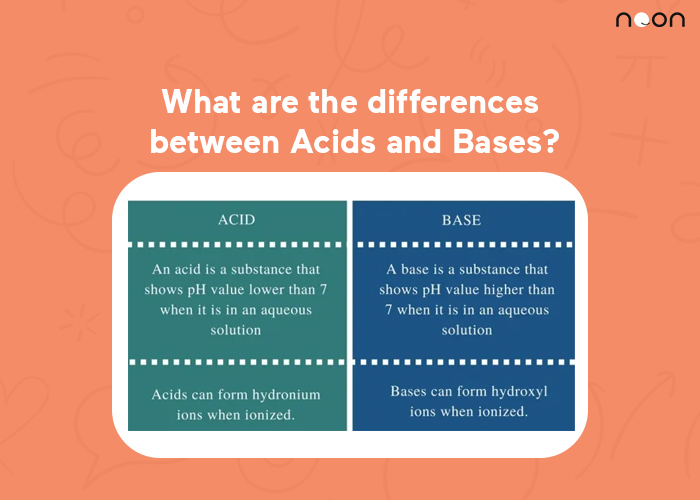
Difference between Acid and Bases
Acids and bases are two important concepts in chemistry. The differences between acids and bases can be summarized as follows:
– Acids increase the concentration of H+ ions in a solution, while bases decrease the concentration of H+ ions in a solution.
– Acids are typically sour in taste, while bases are typically bitter in taste.
– Acids turn litmus paper red, while bases turn litmus paper blue.
Conclusion
Acids and bases are two distinct chemical compounds that have very different properties. Acids are characterized by their sour taste, corrosive action, and ability to conduct electricity, while bases can be recognized by their bitter taste, slippery feel when dissolved in water, and high pH value.
Understanding the differences between acids and bases is important for working with many types of materials in science labs or industries.
With a better understanding of these compounds’ characteristics, we can avoid any potential hazards associated with them.
Noon is an amazing application that can help you learn more about other subjects and ace your next exam. With over 10,000 lectures on different subjects, you can learn from the best teachers from across the globe.
The ultimate teacher’s assistant tool for e-learning, Noon provides teachers with tools for online teaching, including video streams online and high-quality videos with interactive classrooms.
This makes it easy for you to get the most out of your learning experiences and improve your grades.
Download the Noon app today to improve your grades!