Have you ever wondered why some atoms and molecules are electrically charged while others are not? The answer lies in the difference between cations and anions.
In this blog post, we’ll explore what are cations and anions, the differences between cations and anions as well as how they differ from neutral particles. So, stay tuned!
What is a Cation?
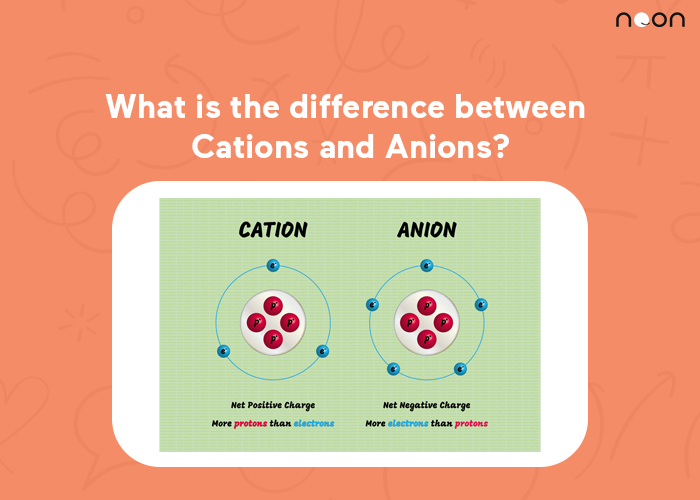
A cation is an atom that has lost one or more electrons and has a positive charge. Cations are attracted to anions, which are atoms that have gained one or more electrons and have a negative charge.
What is an Anion?
An anion is a negatively charged ion. It is formed when an atom loses one or more electrons. The name of the anion is derived from the word “anode,” which is the electrode at which oxidation occurs.
Cation vs Anion
In general, cations are positively charged ions while anions are negatively charged ions. The terms “cation” and “anion” stem from the Greek word for going up and down, respectively. In water solutions, cations move toward the cathode (negative pole) while anions move toward the anode (positive pole).
One of the most significant differences between cations and anions is their size.
Cations are always smaller than the atom or molecule they came from while anions are larger. This is due to the number of electrons in the outermost orbital.
Cations have lost one or more electrons so they have a smaller electron cloud. Anions have gained one or more electrons so they have a larger electron cloud.
Another difference between cations and anions is that cations are always metal elements while anions can be either metal or nonmetal elements. This is because it takes much more energy to remove an electron from a metal than it does to remove one from a nonmetal.
As a result, metals tend to form cations while nonmetals tend to form anions.
Examples of cations and anions
Ionic compounds are made up of cations and anions. Cations are positively charged ions, while anions are negatively charged ions. Ionic compounds are held together by the electrostatic attraction between the cations and anions.
Common cations include sodium (Na+), potassium (K+), magnesium (Mg2+), calcium (Ca2+), and iron (Fe3+).
Common anions include chloride (Cl-), bromide (Br-), iodide (I-), oxide (O2-), and sulfide (S2-).
Conclusion
In summary, cations and anions are ions with opposite charges. Cations have a positive charge while anions have a negative charge.
These ions are found in many of the substances that make up our world and play a vital role in maintaining a balance between positively and negatively charged particles.
Knowing what these two different types of ions are can help you understand why certain reactions occur when they come into contact with each other and how to best manage any potential chemical hazards.
Noon is an online learning platform that offers over 10,000 lectures on different subjects. You can learn from the best teachers from across the globe with this app.
Ultimate Teacher’s Assistant Tool for E-learning: Noon provides teachers with tools for online teaching, it is an online study app for teachers with video streams online, and high-quality videos with interactive classrooms.
With the Noon app, you can access lectures at any time and from anywhere. This makes learning more convenient and flexible for busy students. So, download the Noon app for a fun learning experience!