Have you ever wondered what the difference is between elements, compounds, and mixtures? It’s a question that has been asked many times over, and it’s important to understand the differences between them in order to make sense of how chemicals interact with each other.
In this blog post, we will explore the fundamental differences between elements, compounds, and mixtures.
By the end of this article, you should have a better understanding of these three concepts and how they are different and you will know how to teach elements compounds and mixtures to your fellow students.
What are Elements Compounds and Mixtures?
Elements, compounds, and mixtures are all matter. They are made up of atoms, which are the smallest particles of an element that have the chemical properties of that element.
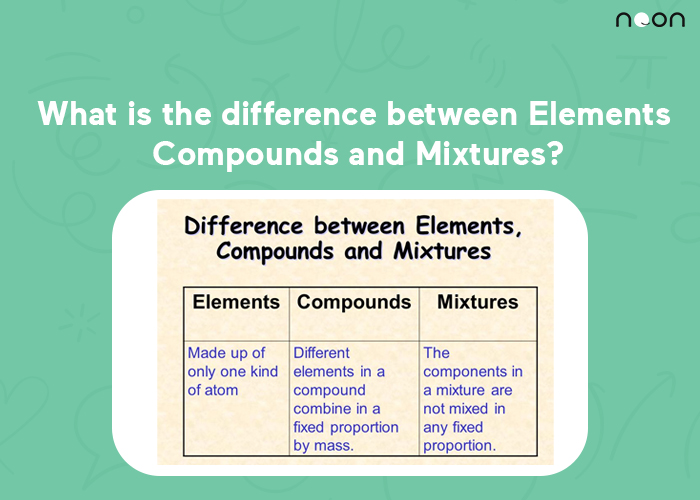
The elements are the simplest form of matter and cannot be broken down into anything simpler. The periodic table is a list of all the known elements. There are currently 118 elements on the periodic table, with new ones being discovered all the time.
Compounds are formed when two or more elements are chemically combined. The molecules of a compound are held together by chemical bonds, which can be ionic (electrostatic) or covalent (sharing electrons). Water (H2O) is a common compound.
Mixtures are created when two or more substances are physically combined and not chemically bonded. The components of a mixture can be separated by physical means, such as filtration or evaporation. Air is a mixture of nitrogen and oxygen gas.
Difference between elements Compounds and Mixtures
Elements, compounds, and mixtures are all substances that are made up of atoms. The difference between them is in the way the atoms are arranged.
In an element, the atoms are arranged in a simple, repeating pattern. There are only 118 elements on the periodic table, and they cannot be broken down into simpler substances by chemical means.
A compound is a substance made up of two or more different elements that have been chemically joined together. Compounds have properties that are different from the elements they are made from.
For example, water is a compound made from the elements hydrogen and oxygen. It has properties that are different from those of hydrogen and oxygen, such as being liquid at room temperature.
Mixtures are substances made up of two or more different elements or compounds that have been physically combined, but not chemically joined together. Mixtures can be either homogeneous or heterogeneous.
A homogeneous mixture is one in which the composition is uniform throughout; an example is salt water, where you can’t see the individual salt grains anymore because they’re completely dissolved in the water.
A heterogeneous mixture is one in which the composition is not uniform; an example is a bowl of cereal, where you can see both the cereal pieces and the milk they’re floating in.
Conclusion
The distinction between elements, compounds, and mixtures is an important one to understand. Elements are the most basic forms of matter and contain only one kind of atom.
Compounds consist of two or more different kinds of atoms joined together, while mixtures can be made up of multiple elements and/or compounds that have been combined without any chemical bonding taking place.
Understanding these differences will help you gain a better understanding of chemistry and how the world works around us!
The Noon app is the perfect tool for students who want to learn more about other subjects and ace their next exam.
With over 10,000 lectures on different subjects available, students can learn from the best teachers from all around the globe.
Noon provides professional teachers with online teaching tools, including video streaming, high-quality videos, and interactive classrooms. This makes it the ultimate teacher’s assistant tool for e-learning.
Not only does it provide an easy way for teachers to deliver lectures online, but it also ensures that students have access to quality education resources. So, download the Noon app now!