What are halogens?
The name “halogen” comes from the Greek words for “salt-producing.”
A halogen is a chemical element with properties that are intermediate between those of metals and nonmetals. Halogens are found in the periodic table in group 17, and include fluorine, chlorine, bromine, iodine, and astatine.
Halogens are highly reactive and form compounds with most other elements. They are found in a variety of minerals, including salt deposits. Halogens are used in a variety of industrial applications, including the manufacture of plastics, dyes, and electronic components. They are also used as disinfectants and bleaching agents.
The halogens are a group of five nonmetallic elements that include fluorine, chlorine, bromine, iodine, and astatine. They all have seven electrons in their outermost energy level, giving them similar chemical properties. However, there is one key difference between the halogens: electronegativity.
What is Electronegativity?
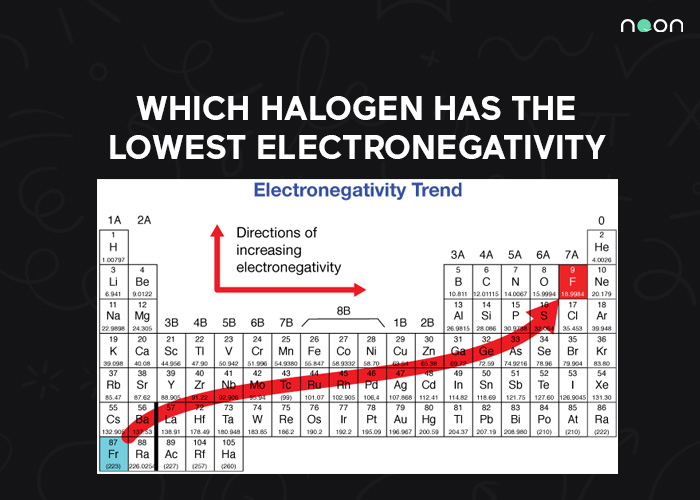
Electronegativity is a measure of an atom’s ability to attract electrons to itself. Fluorine has the highest electronegativity of any element, while astatine has the lowest.
This means that fluorine is more likely to form covalent bonds with other atoms, while astatine is more likely to form ionic bonds. As a result, the halogens can be divided into two groups: highly electronegative and less electronegative.
Highly Electronegative Halogens
Highly electronegative halogens are elements that have a very strong affinity for electrons. As a result, they are highly reactive and tend to form compounds with other elements. Halogens are found in Group 17 of the periodic table, and include fluorine, chlorine, bromine, iodine, and astatine. All of these elements are highly electronegative, and as a result, they are all highly reactive.
For example, fluorine is the most electronegative element on the periodic table, and as a result, it is extremely corrosive. It is so reactive that it will even react with water to form hydrofluoric acid. Highly electronegative halogens are important in many industrial and commercial applications, and their reactivity makes them essential ingredients in many products.
Which Halogen Has The Lowest c?
Astatine is the least electronegative of the five elements. This means that it is the least likely to form negative ions when it bonds with other elements. Instead, astatine tends to form positive ions or cations. This is because astatine has a relatively small atomic radius, which gives it high ionization energy.
As a result, astatine often forms compounds with metals rather than with non-metals. Although it is less electronegative than the other halogens, astatine still has a higher electronegativity than most of the other elements in the periodic table. This is due to the fact that astatine has a high atomic weight and a small atomic radius.
As a result, astatine is still able to form strong bonds with other atoms.
Uses and Application of Halogens
Halogens are often used in industrial processes such as water treatment and metal production. Halogens also have a strong tendency to gain electrons, which gives them unique electrical properties.
For instance, when added to glass, halogens can make the glass conductive. This is why iodine is often used in making LCD screens and other electronic devices. In general, halogens are versatile elements with a wide range of applications.
- Fluorine is used in many industries, including aluminum production and semiconductor manufacturing.
- Chlorine is used as a disinfectant and bleaching agent, and it is also an essential component of many household cleaners.
- Bromine is used in fire retardants and pesticides, while iodine is used in medicine and photography.
- Astatine has the highest reactivity of all the halogens, and as a result, it has few commercial uses.
However, it does have a variety of scientific applications, including cancer treatment. Overall, halogens are versatile elements that play an important role in many different fields.