Solution:
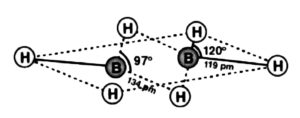
(a) Diborane
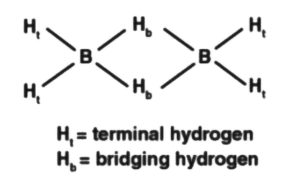
Two boron and four-terminal molecules of hydrogen 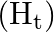 lie one way, while the other two connecting particles of hydrogen
lie one way, while the other two connecting particles of hydrogen 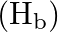 are in the surface opposite to the plane of boron iotas. Once, of the two molecules of hydrogen spanning, one particle of
are in the surface opposite to the plane of boron iotas. Once, of the two molecules of hydrogen spanning, one particle of 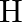 lies over the plane, and the other beneath the plane. The terminal bonds are standard twocentre two-electron
lies over the plane, and the other beneath the plane. The terminal bonds are standard twocentre two-electron 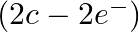 bonds, while the two bridgings
bonds, while the two bridgings 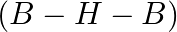 bonds are three-focus two-electron (3c
bonds are three-focus two-electron (3c 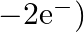 bonds.
bonds.
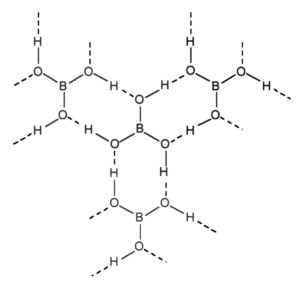
(b) Boric corrosive
Boric corrosive is organized in a layered structure. Each planar unit 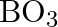 is associated by molecules
is associated by molecules 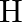 to one another. The
to one another. The 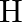 molecules structure a covalent bond with a
molecules structure a covalent bond with a 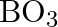 unit while another
unit while another 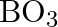 unit frames a hydrogen bond. The dabbed lines, in the given figure, address hydrogen bonds.
unit frames a hydrogen bond. The dabbed lines, in the given figure, address hydrogen bonds.