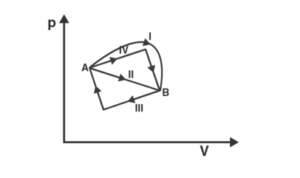
a) change in internal energy is the same in IV and III cases, but not in I and II
b) change in internal energy same in all the four cases
c) work done is maximum in case I
d) work done is minimum in case II
Answer:
The correct options are
b) change in internal energy same in all the four cases
c) work done is maximum in case I
Explanation: Internal energy is a result of a system’s state and is independent of its path. As a result, internal energy will be equal.
