Solution:
Carbon dioxide
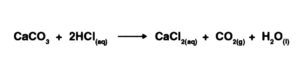
CO2 can be ready in the lab through the activity of weaken hydrochloric corrosive on calcium carbonate. Their response is as per the following:
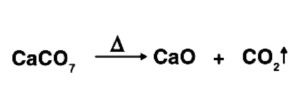
CO2 is industrially ready by warming limestone. The response included is as per the following:
Carbon monoxide
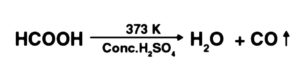
In the lab, CO is ready by the parchedness of formic corrosive with conc. H2SO4, at 373 K. The response included is as per the following:
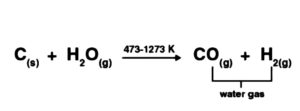
The CO is arranged economically by ignoring steam hot coke: