Solution:
(a) While producing benzoic corrosive from toluene, alcoholic potassium permanganate is utilized as an oxidant because of the given reasons.
(I) In an impartial medium, ![]() ions are created in the response. Because of that, the expense of adding a corrosive or a base can be diminished.
ions are created in the response. Because of that, the expense of adding a corrosive or a base can be diminished.
(ii) ![]() and liquor are homogeneous to one another as they are polar. Liquor and toluene are homogeneous to one another in light of the fact that both are natural mixtures. Responses can continue at a quicker rate in a homogeneous medium contrasted with heterogeneous medium. Consequently, in liquor,
and liquor are homogeneous to one another as they are polar. Liquor and toluene are homogeneous to one another in light of the fact that both are natural mixtures. Responses can continue at a quicker rate in a homogeneous medium contrasted with heterogeneous medium. Consequently, in liquor, ![]() and toluene can respond at a quicker rate
and toluene can respond at a quicker rate
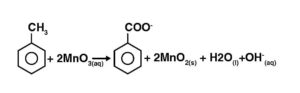
The redox response is as given beneath:
(b) When concentrated ![]() is added to an inorganic combination containing bromide, right off the bat
is added to an inorganic combination containing bromide, right off the bat ![]() is created.
is created.
HBr, a solid lessening specialist, diminishes ![]() to
to ![]() with the development of bromine’s red fume.
with the development of bromine’s red fume.
![]()
![]()
When concentrated ![]() । added to an inorganic combination containing chloride, a sharp smelling gas (HCl) is advanced.
। added to an inorganic combination containing chloride, a sharp smelling gas (HCl) is advanced. ![]() , a frail decreasing specialist, can’t diminish
, a frail decreasing specialist, can’t diminish ![]() to
to ![]() .
.
![]()
