Solution:
A tomic sweep (in pm)
Aluminum
Gallium
In spite of the fact that Ga has more than one shell than Al, it is more modest in size than Al. This is on the grounds that the 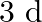 electrons have helpless protecting impact. The protecting impact of d-electrons is exceptionally poor, and the compelling atomic charge experienced in gallium by the valence electrons is undeniably more than in Al.
electrons have helpless protecting impact. The protecting impact of d-electrons is exceptionally poor, and the compelling atomic charge experienced in gallium by the valence electrons is undeniably more than in Al.
