According to the Bohr model, the energies of ![]() and
and ![]() will be fairly near because each have one electron, much like the hydrogen atom, and the nucleus is four times heavier than the H-atoms. As a result, the stability of these atoms, as well as their energy levels, remains near to that of the H atom.
will be fairly near because each have one electron, much like the hydrogen atom, and the nucleus is four times heavier than the H-atoms. As a result, the stability of these atoms, as well as their energy levels, remains near to that of the H atom.
Imagine removing one electron from 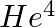 and
and 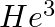 . Their energy levels, as worked out on the basis of Bohr model will be very close. Explain why.
. Their energy levels, as worked out on the basis of Bohr model will be very close. Explain why.
Imagine removing one electron from 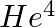 and
and 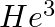 . Their energy levels, as worked out on the basis of Bohr model will be very close. Explain why.
. Their energy levels, as worked out on the basis of Bohr model will be very close. Explain why.
