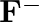The electronic configuration of the Hydrogen atom (in its ground state) ![]() . The single negative charge on this atom indicates that it has gained an electron. Hence, the electronic configuration of
. The single negative charge on this atom indicates that it has gained an electron. Hence, the electronic configuration of ![]()
(b) ![]() ion
ion
Electron configuration of ![]() Here, the +ve charge indicates the loss of an electron.
Here, the +ve charge indicates the loss of an electron. ![]()
Electronic configuration of ![]()
(c) ![]() ion
ion
Electronic configuration of 0 xygen ![]() . The ‘- 2 ‘ charge suggests that it has gained 2 electrons.
. The ‘- 2 ‘ charge suggests that it has gained 2 electrons. ![]()
Electronic configuration of ![]() ion
ion ![]()
(d) ![]() ion
ion
Electronic configuration of Fluorine ![]() . The species has gained one electron (accounted for by the
. The species has gained one electron (accounted for by the ![]() charge).
charge). ![]() Electron configuration of
Electron configuration of ![]() ion
ion ![]()
(I)Write the electronic configurations of the following ions:(a) 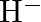 (b)
(b) 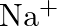 (c)
(c) 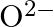 (d)
(d) 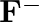
(I)Write the electronic configurations of the following ions:(a) 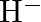 (b)
(b) 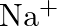 (c)
(c) 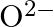 (d)
(d)