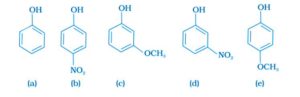
Option (ii) is the answer.
The sequence of decreasing acid strength is b>d>a>c>e, with p-nitrophenol being the most acidic and p-methoxy phenol being the least acidic. The acidity is greatest when an electron withdrawing group is parallel to the OH group. The acidity is lowest when an electron releasing group is opposite the OH group.
