has a high dipole moment because Oxygen is highly electronegative.
This attracts the electron from Hydrogen towards it with greater charge.
Therefore,
has a high dipole moment. 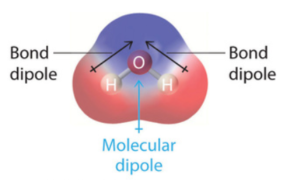
has a high dipole moment because Oxygen is highly electronegative.
This attracts the electron from Hydrogen towards it with greater charge.
Therefore,
has a high dipole moment. 