The results obtained have been tabulated as follows
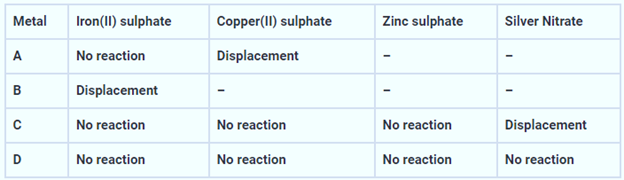
Use the table above to answer the following questions about metals A, B, C and D.
- Which is the most reactive metal?
- What would you observe if B is added to a solution of Copper (II) sulphate?
- Arrange the metals A, B, C and D in the order of decreasing reactivity.
Answer:
(i) Metal B is the most reactive because it reacts with iron (II) sulphate to form a displacement reaction.
(ii) When metal B is added to copper (II) sulphate solution, a displacement reaction occurs, causing the blue colour of the copper (II) sulphate solution to fade and a reddish-brown copper deposit to form on metal B.
(iii)The most reactive metal is Metal B, which displaces iron from its salt solution. Because it displaces copper from its salt solution, Metal A is less reactive. Metal C is even less reactive because it can only displace silver from its salt solution, while metal D is the least reactive because it can’t displace any metal. As a result, the metals are arranged in decreasing order of reactivity. as B > A > C > D.
