Answer:
Assuming the concentration of ![]() at equilibrium be
at equilibrium be ![]()
The given reaction is:
![]() +
+![]()
Given, intial mol.= ![]() of
of ![]() &
&
![]() of
of ![]()
At equilibrium, the moles will be following of Nitrogen and oxygen, respectively, ![]()
![]()
The moles product is, ![]()
Thus, concentration of each will be,
![]()
The equilibrium constant has a value that is exceedingly tiny. This implies that only tiny quantities are involved. Then,
![]() and
and ![]()
Now substutiting the value,
![Rendered by QuickLaTeX.com K_{c}=\frac{\left[N_{2} O_{(g)}\right]^{2}}{\left[N_{2(g)}\left[O_{2(g)}\right]\right.}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-1543e45fb92e2d92127d479329a21fb3_l3.png)
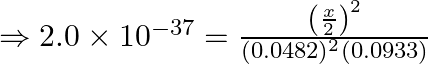
![]()
![]()
![]()
![]()
