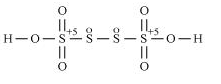Solution:
H2S4O6:
Let x be the oxidation number of S.
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} Oxidation\text{ }no.\text{ }of\text{ }H\text{ }=\text{ }+1 \\ Oxidation\text{ }no.\text{ }of\text{ }O\text{ }=\text{ }-2 \\ ~ \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-08e34f79460baca81851072b7a651d32_l3.png)
Then,
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} 2\left( +1 \right)\text{ }+\text{ }4\left( x \right)\text{ }+\text{ }6\left( -2 \right)\text{ }=\text{ }0 \\ 2\text{ }+\text{ }4x\text{ }-12\text{ }=\text{ }0 \\ 4x\text{ }=\text{ }10 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c42b72a23c8293b1cb7583f915df5bb7_l3.png)
![]()
Oxidation number cannot be fractional. Therefore, S would be present with different oxidation state in molecule.
The oxidation number of two out of the four S atoms is +5 while that of other two atoms is 0.