Answer:
Given information in the question,
![]()
As we know that,
![Rendered by QuickLaTeX.com K_{c}=\frac{\left[S O_{3}\right]^{2}}{\left[S O_{2}\right]^{2}\left[O_{2}\right]}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-8e3e46a26e87963f7bf0449c4897ff7f_l3.png)
Thus, evaluating by substiuting the value of given parameters,
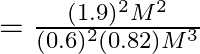
![]() (approximately)
(approximately)
Hence, ![]() for the equilibrium is
for the equilibrium is ![]() ,
,
Answer:
Given information in the question,
![]()
As we know that,
![Rendered by QuickLaTeX.com K_{c}=\frac{\left[S O_{3}\right]^{2}}{\left[S O_{2}\right]^{2}\left[O_{2}\right]}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-8e3e46a26e87963f7bf0449c4897ff7f_l3.png)
Thus, evaluating by substiuting the value of given parameters,
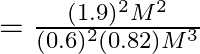
![]() (approximately)
(approximately)
Hence, ![]() for the equilibrium is
for the equilibrium is ![]() ,
,