Solution:
The condition of hybridization of carbon in:
(a) 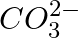
c in 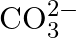 is sp
is sp 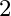 hybridized and is attached to 3 oxygen iotas.
hybridized and is attached to 3 oxygen iotas.
(b) Diamond
Every precious stone carbon is hybridized by sp 3 and is bound to 4 extra carbon iotas.
(c) Graphite
Every carbon iota in graphite is hybridized with sp 2 and is bound to 3 different molecules of carbon.
