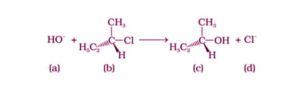
Option (i) and (iii) are the answers.
The SN1 mechanism is used in the given reaction. The production of carbocation is a gradual process in the SN1 mechanism. As a result, the pace of reaction is determined by the concentration of the reactant (ii). As a result, the rate of reaction is determined only by the concentration of (ii), and the molecularity of the reaction is one.
