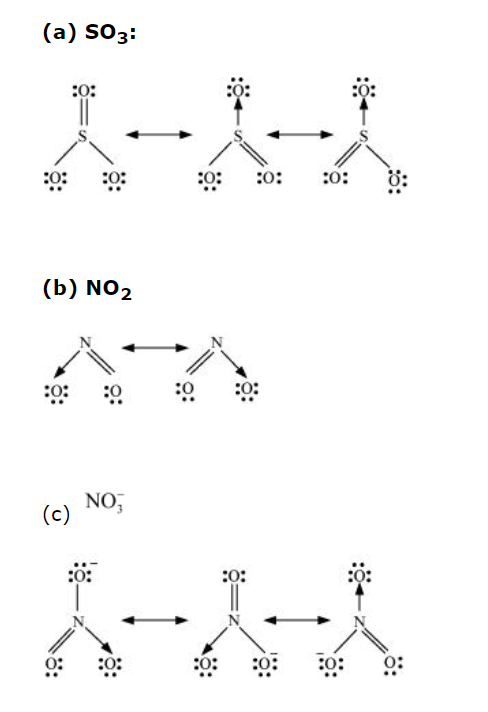
Solution:
Resonance is the phenomenon that allows a molecule to be expressed in multiple ways, none of which fully explain the molecule’s properties. The molecule’s structure is called a resonance hybrid.
The resonating structures must have the same atomic position, number of paired and unpaired electrons, and energy. Resonance structures: