Lattice energy is the name given to the total potential energy of all the forces between atoms in a crystalline solid. It is an essential consideration in predicting solids’ stability and physical properties.
This blog post will discuss what lattice energy is and what it depends upon. We will also explore some of its practical applications. So, if you’re interested in learning more about this fascinating topic, keep reading!
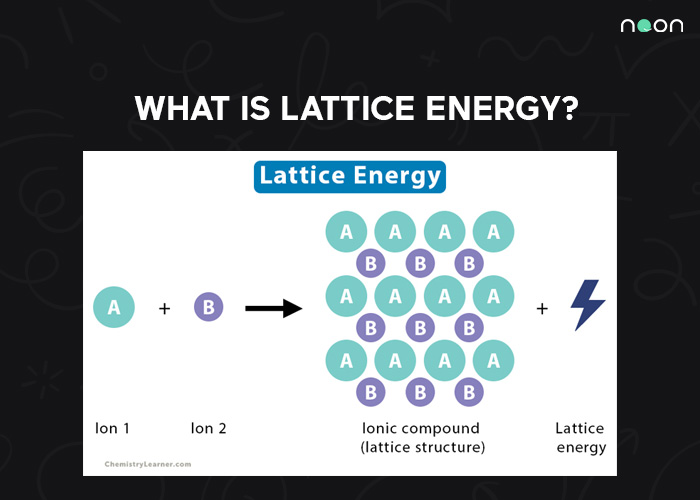
What is Lattice Energy?
Lattice energy is released when oppositely charged ions are brought together to form an ionic solid. This energy is due to the attraction between the positive and negative charges on the ions.
The lattice energy of a compound can be determined by looking at the strength of the ionic bond between the atoms in the compound. The stronger the bond, the higher the lattice energy. The lattice energy can also be affected by the size of the ions. Smaller ions have higher lattice energy than larger ions because they can get closer together, resulting in a stronger attraction.
Additionally, the lattice energy is influenced by the charge on the ions. Ions with a higher charge have higher lattice energy because they have a more potent force of attraction.
Finally, the lattice energy is affected by the solvent in which the compound is dissolved. For example, water has a higher dielectric constant than other solvents, reducing the attractive force between ions, resulting in lower lattice energy. By understanding how these factors influence lattice energy, chemists can predict how strongly compounds will bind together and expect how difficult it will be to break them apart.
Lattice energy trend
Trends in lattice energy can be used to predict the stability of ionic compounds. For example, compounds with high lattice energies are typically more stable than those with low ones. This is because it takes more energy to break apart a compound with high lattice energy than one with low lattice energy. As a result, compounds with high lattice energies are less likely to dissociate into their ions. Consequently, they are more likely to be stable at higher temperatures.
What does lattice energy depends upon?
The lattice energy of an ionic compound is determined by the sum of the radii of the cation and anion. The larger the radius, the greater the lattice energy. Cations with large radii have a small charge density and are more polarizable than cations with small radii.
Anions with small radii have a high charge density and are less polarizable than anions with large radii. The lattice energy of an ionic compound increases as the cation radius decreases and the anion radius increases.
Conclusion
In order to ace your exams, it’s important to understand the trends in lattice energy. By understanding how the lattice energy of an ionic compound is determined, you can predict the stability of different compounds. The larger the radius of the cation and anion, the greater the lattice energy and vice versa. Consequently, compounds with high lattice energies are more stable than those with low ones. At Noon Academy we can help you master these concepts and achieve academic success. Join us today!



