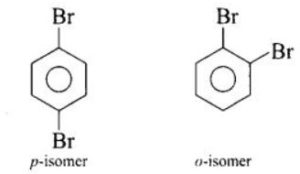
Because the symmetry of p-dibromobenzene allows the molecule to fit better in a crystal lattice, it has a higher melting point. As a result, breaking the bonds between the molecules needs a greater temperature, resulting in a higher melting point.

Because the symmetry of p-dibromobenzene allows the molecule to fit better in a crystal lattice, it has a higher melting point. As a result, breaking the bonds between the molecules needs a greater temperature, resulting in a higher melting point.