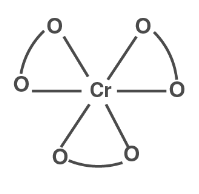
( i ) In [ Cr(C2O4)3] 3− no geometric isomers are present because it is a bidentate ligand.
( ii ) In [ Co( NH3)3 Cl3 ]two isomers are possible.
( i ) In [ Cr(C2O4)3] 3− no geometric isomers are present because it is a bidentate ligand.

( ii ) In [ Co( NH3)3 Cl3 ]two isomers are possible.