Mean free path is given as ![]()
Collision frequency is given as ![]()
Successive collision time ≅ 500 x (Collision time)
Pressure inside the cylinder containing nitrogen will be,
P = ![]()
Temperature inside the cylinder is given as T = 170 C = 290 K
Radius of a nitrogen molecule is given as r = 1.0 Å = 1 x 1010 m
Diameter will be ![]()
Molecular mass of nitrogen is known as ![]()
The root mean square speed of nitrogen is given by the expression,
![]()
Where,
R = universal gas constant having value ![]()
Hence,
![]()
On calculation, we get,
= 508.26 m/s
The mean free path (l) is given by relation:
![]()
Where,
![]() is the Boltzmann constant having value
is the Boltzmann constant having value ![]()
So,
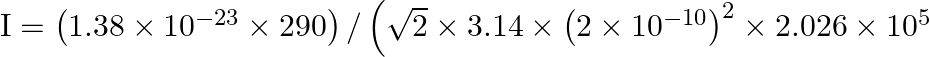
We get,
![]()
Collision frequency ![]()
![]()
On calculation,
![]()
On calculation, we get
![]()
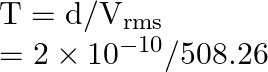
On further calculation, we get
![]()
Time taken between successive collisions:
![]()
![]()
Hence,
![]()
On calculation, we get,
= 500
As a result, the time taken between successive collisions is 500 times the time taken for a collision.
