a) Consider the circuit in the figure. How much energy is absorbed by electrons from the initial state of no current to the state of drift velocity? b) Electrons give up energy at the rate of RI2 per second to the thermal energy. What time scale would one associate with energy in problem a) n = no of electron/volume = 1029/m3, length of circuit = 10 cm, cross-section = A = 1mm2
a) Current is given as I = V/R from the Ohm’s law Therefore, I = 1A But, I = ne Avd vd = I/neA When the values for the above parameters are substituted, vd = 1/1.6 × 10-4 m/s The KE = (KE of one...
In an experiment with a potentiometer, VB = 10V. R is adjusted to be 50 Ω. A student wanting to measure voltage E1 of a battery finds no null point possible. He then diminishes R to 10 Ω and is able to locate the null point on the last segment of the potentiometer. Find the resistance of the potentiometer wire and potential drop per unit length across the wire in the second case.
Equivalent resistance of the potentiometer = 50 Ohm + R’ Equivalent voltage across the potentiometer = 10 V Current through the main circuit I = 10/(50 Ohms +R’) Potential difference across wire of...
A room has AC run for 5 hours a day at a voltage of 220V. The wiring of the room consists of Cu of 1 mm radius and a length of 10 m. Power consumption per day is 10 commercial units. What fraction of it goes in the joule heating in wires? What would happen if the wiring is made of aluminium of the same dimensions?
Power consumption in a day = 10 units Power consumption per hour = 2 units Power consumption = 2 units = 2 kW = 2000 J/s Power consumption in resistors, P = VI Which gives I = 9A We know that...
Two cells of voltage 10V and 2V and internal resistances 10Ω and 5Ω respectively are connected in parallel with the positive end of the 10V battery connected to the negative pole of 2V battery. Find the effective voltage and effective resistance of the combination.
Kirchhoff’s law is applied at c, I1 = I + I2 Kirchhoff’s law is applied at efbae, 10 – IR – 10I2 = 0 10 = IR + 10I1 Kirchhoff’s law is applied at cbadc, -2-IR+5I2 = 0 2 = 5I2- RI I2 = I1 – I...
Suppose there is a circuit consisting of only resistances and batteries and we have to double all voltages and all resistances. Show that currents are unaltered.
Reff is the equivalent internal resistance of the battery Veff is the equivalent voltage of the battery Using Ohm’s law, I = Veff/(Reff + R) When the resistance and effective voltage are increased...
(i) Consider a thin lens placed between a source (S) and an observer (O). Let the thickness of the lens vary as 2 0 () – α = b wb w, where b is the verticle distance from the pole. w0 is a constant. Using Fermat’s principle i.e. the time of transit for a ray between the source and observer is an extremum, find the condition that all paraxial rays starting from the source will converge at a point O on the axis. Find the focal length.
(ii) A gravitational lens may be assumed to have a varying width of the form w(b) = k1 ln (k2/b) = k1 ln (k2/bmin). Show that an observer will see an image of a point object as a ring about the centre of the lens with an angular radius.
β = √(n-1)k1 u/v / u + v
i) Time taken by the ray to travel from S to P1 is = t1 = √u2 + b2/c Time taken by the ray to travel from P1 to O is = t2 = v/c (1+ ½ b2/v2) Time taken to travel through the lens is =...
An infinitely long cylinder of radius R is made of an unusual exotic material with refractive index –1. The cylinder is placed between two planes whose normals are along the y-direction. The centre of the cylinder O lies along the y-axis. A narrow laser beam is directed along the y-direction from the lower plate. The laser source is at a horizontal distance x from the diameter in the y-direction. Find the range of x such that light emitted from the lower plane does not reach the upper plane.
The refractive index of the cylinder is -1 and is placed in the air of μ = 1 AB is incident at B to the cylinder such that θr will be negative θ1= θi = θr Total deviation of the outcoming ray is...
If light passes near a massive object, the gravitational interaction causes a bending of the ray. This can be thought of as happening due to a change in the effective refractive index of the medium given by n(r) = 1 + 2 GM/rc2 where r is the distance of the point of consideration from the centre of the mass of the massive body, G is the universal gravitational constant, M the mass of the body and c the speed of light in vacuum. Considering a spherical object find the deviation of the ray from the original path as it grazes the object.
n(r) = 1 + 2GM/rc2
The mixture a pure liquid and a solution in a long vertical column (i.e, horizontal dimensions << vertical dimensions) produces diffusion of solute particles and hence a refractive index gradient along the vertical dimension. A ray of light entering the column at right angles to the vertical deviates from its original path. Find the deviation in travelling a horizontal distance d << h, the height of the column.
Let the height of the long vertical column with transparent liquid be h and dx be the thickness The angle at which the ray AB enters is θ Let y be the new height of the liquid (θ + d θ) is the...
Show that for a material with refractive index µ ≥ 2 , light incident at any angle shall be guided along a length perpendicular to the incident face. Answer:
Let the refractive index of the rectangular slab be μ ≥ √2. μ = 1/sin ic sin ic > 1/ μ cos r ≥ 1/ μ sin i/sin r = μ From Snell’s law Sin I = μ sin r i = 90o 1 + 1 ≤ μ2 2 ≤ μ2 Taking the square...
A myopic adult has a far point at 0.1 m. His power of accomodation is 4 diopters. (i) What power lenses are required to see distant objects? (ii) What is his near point without glasses? (iii) What is his near point with glasses? (Take the image distance from the lens of the eye to the retina to be 2 cm.)
i) Power lenses are required to see distant objects 1/f = 1/v – 1/u 1/f = 1/10 f = -10 cm = -0.1 m P = 1/f P = 1/(-0.1) P = -10 diopter ii) When no corrective lens used Pn = Pf + Pa u = -10 cm =...
A jar of height h is filled with a transparent liquid of refractive index µ. At the centre of the jar on the bottom surface is a dot. Find the minimum diameter of a disc, such that when placed on the top surface symmetrically about the centre, the dot is invisible.
tan ic d/2/h ic = d/2h d = 2h tan ic d = 2h ×1/√μ2 – 1
In many experimental set-ups the source and screen are fixed at a distance say D and the lens is movable. Show that there are two positions for the lens for which an image is formed on the screen. Find the distance between these points and the ratio of the image sizes for these two points.
u = -x1 v = +(D – x1) 1/D – x1 – 1/(-x1) = 1/f u = -x2 v = +(D – x2) 1/D – x2 – 1/(-x2) = 1/f D = x1 + x2 d = x2 – x1 x1 = D – d/2 D – x1 = D + d/2 u = D/2 + d/2 v = D/2 – d/2 m1 = D – d/D + d m2/m1...
A thin convex lens of focal length 25 cm is cut into two pieces 0.5 cm above the principal axis. The top part is placed at (0,0) and an object placed at (–50 cm, 0). Find the coordinates of the image.
1/v = 1/u + 1/f = 1/-50 + 1/25 = 1/50 v = 50 cm Magnification is m = v/u = -50/50 = -1 Therefore, the coordinates of the image are (50 cm, -1 cm) ...
A circular disc of radius ‘R’ is placed co-axially and horizontally inside an opaque hemispherical bowl of radius ‘a’. The far edge of the disc is just visible when viewed from the edge of the bowl. The bowl is filled with transparent liquid of refractive index µ and the near edge of the disc becomes just visible. How far below the top of the bowl is the disc placed?
Distance at which the bowl should be placed in the disc is given as: d = μ(a2 – b2)/√(a + r)2 – μ(a – r)2
A short object of length L is placed along the principal axis of a concave mirror away from focus. The object distance is u. If the mirror has a focal length f, what will be the length of the image? You may take L << |v-f|
The mirror formula is 1/v + 1/u = 1/f u is the object distance v is the image distance du = |u1 – u2| = L Differentiating on the both sides we get, dv/v2 = -du/u2 v/u = f/u-f du = L, therefore,...
For a glass prism (µ = √3 ) the angle of minimum deviation is equal to the angle of the prism. Find the angle of the prism.
μ = sin[(A + δm)/2]/sin (A/2)
Three immiscible liquids of densities d1 > d2 > d3 and refractive indices µ1 > µ2 > µ3 are put in a beaker. The height of each liquid column is h/3. A dot is made at the bottom of the beaker. For near-normal vision, find the apparent depth of the dot. Answer:
An unsymmetrical double convex thin lens forms the image of a point object on its axis. Will the position of the image change if the lens is reversed?
The near vision of an average person is 25cm. To view an object with an angular magnification of 10, what should be the power of the microscope?
Will the focal length of a lens for red light be more, same or less than that for blue light?
An astronomical refractive telescope has an objective of focal length 20m and an eyepiece of focal length 2cm.(a) The length of the telescope tube is 20.02m. (b) The magnification is 1000. (c) The image formed is inverted. (d) An objective of a larger aperture will increase the brightness and reduce chromatic aberration of the image.
Answer: (a) The length of the telescope tube is 20.02m. (b) The magnification is 1000. (c) The image formed is inverted. ...
A magnifying glass is used, as the object to be viewed can be brought closer to the eye than the normal near point. This results in
(a) a larger angle to be subtended by the object at the eye and hence viewed in greater detail.
(b) the formation of a virtual erect image.
(c) increase in the field of view.
(d) infinite magnification at the near point.
Answer: (a) a larger angle to be subtended by the object at the eye and hence viewed in greater detail. (b) the formation of a virtual erect image. ...
Between the primary and secondary rainbows, there is a dark band known as Alexandar’s dark band. This is because
(a) light scattered into this region interfere destructively.
(b) there is no light scattered into this region
(c) light is absorbed in this region.
(d) angle made at the eye by the scattered rays with respect to the incident light of the sun lies between approximately 42° and 50°.
Answer: (a) light scattered into this region interfere destructively. (d) angle made at the eye by the scattered rays with respect to the incident light of the sun lies between approximately 42° and...
A rectangular block of glass ABCD has a refractive index 1.6. A pin is placed midway on the face AB. When observed from the face AD, the pin shall
(a) appear to be near A.
(b) appear to be near D.
(c) appear to be at the centre of AD.
(d) not be seen at all.
Answer: (a) appear to be near A. (d) not be seen at all. The pin will appear to be near A as long as the angle of incidence on AD of the ray emerging from the pin is smaller...
Consider an extended object immersed in water contained in a plane trough. When seen from close to the edge of the trough the object looks distorted because
(a) the apparent depth of the points close to the edge is nearer the surface of the water compared to the points away from the edge.
(b) the angle subtended by the image of the object at the eye is smaller than the actual angle subtended by the object in the air.
(c) some of the points of the object far away from the edge may not be visible because of total internal reflection.
(d) water in a trough acts as a lens and magnifies the object.
Answer: (a) the apparent depth of the points close to the edge is nearer the surface of the water compared to the points away from the edge. (b) the angle subtended by the image of the object at the...
There are certain material developed in laboratories which have a negative refractive index (Fig. 9.3). A ray incident from the air (medium 1) into such a medium (medium 2) shall follow a path given by
Answer: (a) The speed of the car in the rear is 65 km h–1. Negative refractive index materials react to Snell's law in the exact opposite direction. When a...
A car is moving with at a constant speed of 60 km h–1 on a straight road. Looking at the rearview mirror, the driver finds that the car following him is at a distance of 100 m and is approaching with a speed of 5 km h –1. In order to keep track of the car in the rear, the driver begins to glance alternatively at the rear and side mirror of his car after every 2 still the other car overtakes. If the two cars were maintaining their speeds, which of the following statement (s) is/are correct?
(a) The speed of the car in the rear is 65 km h–1.
(b) In the side mirror, the car in the rear would appear to approach with a speed of 5 km h–1 to the driver of the leading car.
(c) In the rearview mirror the speed of the approaching car would appear to decrease as the distance between the cars decreases.
(d) In the side mirror, the speed of the approaching car would appear to increase as the distance between the cars decreases.
Answer: (d) In the side mirror, the speed of the approaching car would appear to increase as the distance between the cars decreases.
The optical density of turpentine is higher than that of water while its mass density is lower. The figure shows a layer of turpentine floating over water in a container. For which one of the four rays incident on turpentine in the figure, the path shown is correct?
a) 1
b) 2
c) 3
d) 4
Answer: b) 2 When light travels from (optically) rarer medium air to optically denser medium turpentine, it bends towards the normal, i.e., θ1 >...
The direction of a ray of light incident on a concave mirror as shown by PQ while directions in which the ray would travel after reflection is shown by four rays marked 1, 2, 3, and 4. Which of the four rays correctly shows the direction of reflected ray?
a) 1
b) 2
c) 3
d) 4
Answer: b) 2 After reflection, the ray PQ of light that passes through focus F and strikes the concave mirror should become parallel to the primary...
The phenomena involved in the reflection of radiowaves by ionosphere is similar to
a) reflection of light by a plane mirror
b) total internal reflection of light in the air during a mirage
c) dispersion of light by water molecules during the formation of a rainbow
d) scattering of light by the particles of air
Answer: b) total internal reflection of light in the air during a mirage The ionosphere, a layer of the atmosphere, reflects radio waves, allowing them to reach far-flung portions of the globe....
The radius of curvature of the curved surface of a plano-convex lens is 20 cm. If the refractive index of the material of the lens be 1.5, it will
a) act as a convex lens only for the objects that lie on its curved side
b) act as a concave lens only for the objects that lie on its curved side
c) act as a convex lens irrespective of the side on which the object lies
d) act as a concave lens irrespective of the side on which the object lies
Answer: c) act as a convex lens irrespective of the side on which the object lies
You are given four sources of light each one providing a light of a single colour- red, blue, green, and yellow. Suppose the angle of refraction for a beam of yellow light corresponding to a particular angle of incidence at the interface of two media is 90o. Which of the following statements is correct if the source of yellow light is replaced with that of other lights without changing the angle of incidence?
a) the beam of red light would undergo total internal reflection
b) the beam of red light would bend towards normal while it gets refracted through the second medium
c) the beam of blue light would undergo total internal reflection
d) the beam of green light would bend away from the normal as it gets refracted through the second medium
Answer: c) the beam of blue light would undergo total internal reflection
A passenger in an aeroplane shall
a) never see a rainbow
b) may see a primary and a secondary rainbow as concentric circles
c) may see a primary and a secondary rainbow as concentric arcs
d) shall never see a secondary rainbow
Answer: b) may see a primary and a secondary rainbow as concentric circles As an aeroplane flies higher in the sky, passengers may notice a primary and secondary rainbow in the form of concentric...
An object approaches a convergent lens from the left of the lens with a uniform speed 5 m/s and stops at the focus. The image
a) moves away from the lens with a uniform speed 5 m/s
b) moves away from the lens with a uniform acceleration
c) moves away from the lens with a non-uniform acceleration
d) moves towards the lens with a non-uniform acceleration
Answer: c) moves away from the lens with a non-uniform acceleration In our case, the object approaches a convergent lens from the left at a uniform speed of 5 m/s, causing the image to travel away...
A short pulse of white light is incident from air to a glass slab at normal incidence. After travelling through the slab, the first colour to emerge is
a) blue
b) green
c) violet
d) red
Answer: d) Red The relation v = fλ describes the velocity of a wave. The frequency of light does not change when it travels from one medium to another. As a result, the bigger the wavelength, the...
Two cells of same emf E but internal resistance r1 and r2 are connected in series to an external resistor R. What should be the value of R so that the potential difference across the terminals of the first cell becomes zero.
Effective emf of two cells = E + E = 2E Effective resistance = R + r1 + r2 Electric current is given as I = 2E/R+r1+r2 Potential difference is given as V1 – E – Ir1 = 0 Which f=gives R = r1 –...
Let there be n resistors R1……..Rn with Rmax = max(R1……Rn) and Rmin = min(R1…….Rn). Show that when they are connected in parallel, the resultant resistance Rp < Rmin and when they are connected in series, the resultant resistance Rs > Rmax. Interpret the result physically.
The current is represented as I = E/R+nR when the resistors are connected in series. Current is expressed as 10I = E/(R+R/n) when the resistors are connected in parallel....
First, a set of n equal resistors of R each are connected in series to a battery of emf E and internal resistance R. A current I is observed to flow. Then the n resistors are connected in parallel to the same battery. It is observed that the current is increased 10 times. What is ‘n’?
The current is represented as I = E/R+nR when the resistors are connected in series. Current is expressed as 10I = E/(R+R/n) when the resistors are connected in parallel. We get n = 10 by solving...
. A cell of emf E and internal resistance r is connected across an external resistance R. Plot a graph showing the variation of PD across R versus R.
The graphic depiction is as follows: The resistance r is connected across the external resistance R, and E is the cell's emf. V = ER/R+r is the connection between voltage and R....
While doing an experiment with potentiometer it was found that the deflection is one-sided and i) the deflection decreased while moving from one end A of the wire to the end B; ii) the deflection increased, while the jockey was moved towards the end B. i) Which terminal +ve or –ve of the cell E, is connected at X in case
i) and how is E1 related to E?
ii) Which terminal of the cell E1 is connected at X in case ii)?
The positive terminal of cell E1 is linked to E, and E is connected to X. Furthermore, E1 > E ii) cell E1's negative terminal is linked to X.
AB is a potentiometer wire. If the value of R is increased, in which direction will the balance point J shift?
The potential difference across AB dropped as the value of R grew, and therefore the potential gradient across AB decreased. E' = kl is the formula for this....
Power P is to be delivered to a device via transmission cables having resistance Rc. If V is the voltage across R and I the current through it, find the power wasted and how can it be reduced.
P = i2Rc is the power utilised by transmission lines. The resistance of connecting wires is denoted by Rc. P = VI is the formula for calculating power. Power...
Match the example given in Column I with the name of the reaction in Column II
Solution: (i) is e (ii) is d (iii) is a (iv) is b (v) is f (vi) is c
Match the reactions given in Column I with the suitable reagents given in Column II.
Solution: (i) is c (ii) is d (iii) is a (iv) is b
Match the acids given in Column I with their correct IUPAC names given in Column II.
Solution: (i) is b (ii) is e (iii) is d (iv) is a (v) is c
Match the common names given in Column I with the IUPAC names given in Column II
Solution: (i) is d (ii) is e (iii) is a (iv) is b (v) is c
Can Gatterman-Koch reaction be considered similar to Friedel Craft’s acylation? Discuss.
Solution: Both reactions resemble each other. In Friedel Craft’s acylation reaction, an aryl group or benzene is treated with an acid chloride in the presence of anhydrous AlCl3 and corresponding...
Ethylbenzene is generally prepared by acetylation of benzene followed by reduction and not by direct alkylation. Think of a possible reason.
Solution: This is due to the formation of polysubstituted products. To avoid the formation of polysubstituted products Friedel-craft’s alkylation reaction is not used for the preparation of...
Complete the following reaction sequence.
Solution:
Why are carboxylic acids more acidic than alcohols or phenols although all of them have a hydrogen atom attached to an oxygen atom (—O—H)?
Solution: Due to the resonance in carboxylic acids, the negative charge is at the more electronegative oxygen whereas, in alcohols or phenols, the negative charge is on a less electronegative atom....
. Identify the compounds A, B and C in the following reaction.
Solution: Compound A = CH3-MgBr Compound B = CH3-COOH Compound C = CH3COOCH3
Carboxylic acids contain carbonyl group but do not show the nucleophilic addition reaction like aldehydes or ketones. Why?
Solution: The oxygen atom in carbonyl compound pull more shared pair of electron towards itself and so, carbon acquires partial positive charge and oxygen acquires partial negative charge in...
Alkenes and carbonyl compounds both contain a π bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
Solution: Both the compounds carbon atom is attached to the electronegative atom oxygen. Thus the oxygen pulls more shared pair of electron towards them and a partial positive charge will be...
Arrange the following in decreasing order of their acidic strength. Explain the arrangement. C6H5COOH, FCH2COOH, NO2CH2COOH
Solution: NO2CH2COOH > FCH2COOH > C6H5COOH. NO2CH2COOH is most acidic among the given three compounds. Electron withdrawing groups like -NO2, increases the acidity of carboxylic acids by...
Compound ‘A’ was prepared by oxidation of compound ‘B’ with alkaline KMnO4. Compound ‘A’ on reduction with lithium aluminium hydride gets converted back to compound ‘B’. When compound ‘A’ is heated with compound B in the presence of H2SO4 it produces the fruity smell of compound C to which family the compounds ‘A’, ‘B’ and ‘C’ belong to?
Solution: Compound ‘A’ belongs to the carboxylic acid. Compound ‘B’ belongs to alcohol. Compound ‘C’ belongs to an ester group.
What product will be formed on reaction of propanal with 2-methyl propanal in the presence of NaOH? What products will be formed? Write the name of the reaction also.
Solution: When propanal reacts with 2-methyl propanal in the presence of NaOH, the mixture of aldehydes are formed. Both the reactants have an alpha-hydrogen and hence, can undergo cross aldol...
Arrange the following in decreasing order of their acidic strength and give the reason for your answer.
Solution: FCH2COOH > ClCH2COOH > C6H5CH2COOH > CH3COOH > CH3CH2OH. CH3CH2OH is least acidic among the given compounds. C6H5CH2COOH is more acidic than CH3COOH due to the resonance in...
Oxidation of ketones involves carbon-carbon bond cleavage. Name the products formed on oxidation of 2, 5-dimethylhexan-3-one.
Solution: Solution: The products formed on oxidation of 2, 5-dimethylhexan-3-one are the mixtures of ketone and carboxylic acids. Ketone is then further oxidized to carboxylic acids. Overall the...
Name the electrophile produced in the reaction of benzene with benzoyl chloride in the presence of anhydrous AlCl3. Name the reaction also.
Solution: The electrophile produced in the reaction of benzene with benzoyl chloride in the presence of anhydrous AlCl3 is benzoylinium cation. The product formed in this reaction is benzophenone....
Benzaldehyde can be obtained from benzal chloride. Write reactions for obtaining benzyl chloride and then benzaldehyde from it.
SOLUTION: Toluene is first converted to benzal chloride by side-chain chlorination, in presence of Chlorine gas and light. Benzal chloride on hydrolysis at 373K gives benzaldehyde.
Benzaldehyde can be obtained from benzal chloride. Write reactions for obtaining benzyl chloride and then benzaldehyde from it.
Solution:
Write IUPAC names of the following structures.
Solution: (i) Ethane-1,2-dial. (ii) Benzene-1, 4-dicarbaldehyde. (iii) 3-Bromobenzaldehyde.
Give the structure of the following compounds. (i) 4-Nitropropiophenone (ii) 2-Hydroxycyclopentanecarbaldehyde (iii) Phenyl acetaldehyde
Give the IUPAC names of the following compounds
Solution: (i) 3-Phenylprop-2-ene-1-al. (ii) Cyclohexanecarbaldehyde (iii) 3-Oxopentan-1-al (iv) IUPAC name: But-2-enal
Power P is to be delivered to a device via transmission cables having resistance Rc. If V is the voltage across R and I the current through it, find the power wasted and how can it be reduced.
Power consumed by the transmission lines is given as P = i2Rc. Where Rc is the resistance of connecting cables. Power is given as P = VI. The transmission of power takes places either during low...
Arrange the bonds in order of increasing ionic character in the molecules: LiF, 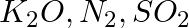 , and
, and 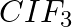 .
.
Solution: The difference in electronegativity between constituent atoms determines the ionic character of a molecule. As a result, the greater the difference, the greater the ionic character of a...
Why are alloys used for making standard resistance coils?
Alloys are used in the making of the standard resistance coils because they have less temperature coefficient of resistance and the temperature sensitivity is also less.
Explain with the help of suitable example polar covalent bond.
Solution: The bond pair of electrons are not shared equally when two unique atoms with different electronegativities join to form a covalent bond. The bond pair is attracted to the nucleus of an...
Define electronegativity. How does it differ from electron gain enthalpy?
Solution: "Electronegativity refers to an atom's ability to attract a bond pair of electrons towards itself in a chemical compound." Sr. No Electronegativity Electron affinity 1 A tendency to...
For wiring in the home, one uses Cu wires or Al wires. What considerations are involved in this?
The main considerations in the selection of the wires is the conductivity of the metal, cost of metal, and their availability.
What is the advantage of using thick metallic strips to join wires in a potentiometer?strips to join wires in a potentiometer?
The advantage of using thick metallic strips is that the resistance of these strips is negligible.
Write the significance/applications of dipole moment.
Solution: There is a difference in electro-negativities of constituents of the atom in a heteronuclear molecule, which causes polarisation. As a result, one end gains a positive charge, while the...
Although both 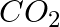 and
and 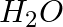 are triatomic molecules, the shape of the
are triatomic molecules, the shape of the 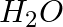 molecule is bent while that of
molecule is bent while that of 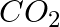 is linear. Explain this on the basis of dipole moment.
is linear. Explain this on the basis of dipole moment.
Solution: $CO_2$ has a dipole moment of 0 according to experimental results. And it's only possible if the molecule's shape is linear, because the dipole moments of the C-O bond are equal and...
What are the advantages of the null-point method in a Wheatstone bridge? What additional measurements would be required to calculate R unknown by any other?
The advantage of a null-point in the Wheatstone bridge is that the resistance of the galvanometer is not affected by the balance point. The R unknown is calculated by using Kirchhoff’s rule.
The relaxation time τ is nearly independent of applied E field whereas it changes significantly with temperature T. First fact is responsible for Ohm’s law whereas the second fact leads to a variation of ρ with temperature. Elaborate why?
Relaxation time is the time interval between two successive collisions of the electrons.It is defined asτ = mean free path/rms velocity of electrons usually, the drift velocity of the electrons is...
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :(iii) Al and N.
Solution: Below is a list of Lewis symbols. To form a cation, a metal atom loses one or more electrons, while a nonmetal atom gains one or more electrons. Ionic bonds are formed between cations and...
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions : (i) K and S (ii) Ca and O
Solution: Below is a list of Lewis symbols. To form a cation, a metal atom loses one or more electrons, while a nonmetal atom gains one or more electrons. Ionic bonds are formed between cations and...
Temperature dependence of resistivity ρ(T) of semiconductors, insulators, and metals is significantly based on the following factors:
a) number of charge carriers can change with temperature T
b) time interval between two successive collisions can depend on T
c) length of material can be a function of T
d) mass of carriers is a function of T
The correct answer is a) number of charge carriers can change with temperature T b) time interval between two successive collisions can depend on T
In a meter bridge, the point D is a neutral point.
a) the meter bridge can have no other neutral point for this set of resistances
b) when the jockey contacts a point on meter wire left of D, current flows to B from the wire
c) when the jockey contacts a point on a meter wire to the right of D, current flows from B to the wire through the galvanometer
d) when R is increased, the neutral point shifts to left
The correct answer is a) the meter bridge can have no other neutral point for this set of resistances c) when the jockey contacts a point on a meter wire to the right of D, current flows from B to...
Write the resonance structures for 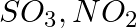 , and
, and 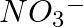
Solution: Resonance is the phenomenon that allows a molecule to be expressed in multiple ways, none of which fully explain the molecule's properties. The molecule's structure is called a resonance...
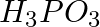 can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing
can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing 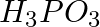 ? If not, give reasons for the same.
? If not, give reasons for the same.
Solution: The positions of the atoms remain constant in canonical forms, but the positions of the electrons change. The positions of atoms change in the given canonical forms. As a result, they...
Explain the important aspects of resonance with reference to the 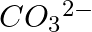 ion.
ion.
Solution: However, while the carbonate ion cannot be represented by a single structure, the properties of the ion can be described by two or more different resonance structures. The actual structure...
Define Bond length.
Solution: Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
How do you express the bond strength in terms of bond order?
Solution: During the formation of a molecule, the extent of bonding that occurs between two atoms is represented by the bond strength of the molecule. As the bond strength increases, the bond...
Although geometries of 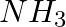 and
and 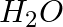 molecules are distorted tetrahedral, bond angle in water is less than that of Ammonia. Discuss.
molecules are distorted tetrahedral, bond angle in water is less than that of Ammonia. Discuss.
Solution: Ammonia's central atom (N) has one lone pair and three bond pairs. In water, the central atom (O) has two lone pairs and two bond pairs. As a result, the two bond pairs repel the two lone...
Discuss the shape of the following molecules using the VSEPR model: 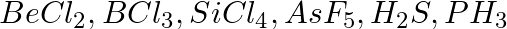
Solution: $BeCl_2$ The central atom does not have a lone pair, but it does have two bond pairs. As a result, its shape is AB2, or linear. $BCl_3$ The central atom has three bond pairs but no lone...
Write the favourable factors for the formation of an ionic bond.
Solution: Ionic bonds are formed when one or more electrons are transferred from one atom to another. As a result, the ability of neutral atoms to lose or gain electrons is required for the...
Define the octet rule. Write its significance and limitations
Solution: “Atoms can combine either by transferring valence electrons from one atom to another or by sharing their valence electrons in order to achieve the closest inert gas configuration by having...
Draw the Lewis structures for the following molecules and ions : 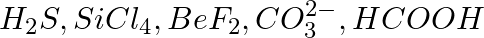
Solution: The lewis dot structures are:
Write Lewis symbols for the following atoms and ions: Sand 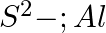 and
and 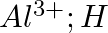 and
and 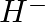
Solution: For S and S2- A sulphur atom has only 6 valence electrons, which is a very small number. As a result, the Lewis dot symbol for the letter S is The presence of a...
The measurement of an unknown resistance R is to be carried out using Wheatstone bridge. Two students perform an experiment in two ways. The first student take R2 = 10Ω and R1 = 5Ω. The other student takes R2 = 1000 Ω and R1 = 500 Ω. In the standard arm, both take R3 = 5 Ω. Both find R = R2/R1 R3 = 10 Ω within errors.
a) the errors of measurement of the two students are the same
b) errors of measurement do depend on the accuracy with which R2 and R1 can be measured
c) if the student uses large values of R2 and R1, the currents through the arms will be feeble. This will make determination of null point accurately more difficult
d) Wheatstone bridge is a very accurate instrument and has no errors of measurement
The correct answer is b) errors of measurement do depend on the accuracy with which R2 and R1 can be measured c) if the student uses large values of R2 and R1, the currents through the arms will be...
Write Lewis dot symbols for atoms of the following elements :e) N f) Br
Solution: Nitrogen atoms have only five valence electrons in total. As a result, the Lewis dot symbol for N is Bromine, because the atom has only seven valence electrons. As a result,...
Write Lewis dot symbols for atoms of the following elements :c) B d) O
Solution: Boron atoms have only three valence electrons, which is a very small number. As a result, the Lewis dot symbols for B are as follows: The oxygen atom has only six valence...
Temperature dependence of resistivity ρ(T) of semiconductors, insulators, and metals is significantly based on the following factors:
a) number of charge carriers can change with temperature T
b) time interval between two successive collisions can depend on T
c) length of material can be a function of T
d) mass of carriers is a function of T
solution:The correct answer is a) number of charge carriers can change with temperature T b) time interval between two successive collisions can depend on T
Consider a simple circuit in the figure.stands for a variable resistance R’.
R’ can vary from R0 to infinity. r is internal resistance of the battery,
a) potential drop across AB is nearly constant as R’ is varied
b) current through R’ is nearly a constant as R’ is varied
c) current I depends sensitively on R’
d) I ≥V/r+R always
solution: The correct answer is a) potential drop across AB is nearly constant as R’ is varied d) I ≥V/r+R always
Kirchhoff’s junction rule is a reflection of
a) conservation of current density vector
b) conservation of charge
c) the fact that the momentum with which a charged particle approaches a junction is unchanged as the charged particle leaves the junction
d) the fact that there is no accumulation of charges at a junction
solution: The correct answer is b) conservation of charge d) the fact that there is no accumulation of charges at a junction
Which of the following characteristics of electrons determines the current in a conductor?
v a) drift velocity alone
b) thermal velocity alone
c) both drift velocity and thermal velocity
d) neither drift nor thermal velocity
solution: The correct answer is a) drift velocity alone
A metal rod of length 10 cm and a rectangular cross-section of 1 cm × 1/2 cm is connected to battery across opposite faces. The resistance will be
a) maximum when the battery is connected across 1 cm × 1/2 cm faces
b) maximum when the battery is connected across 10 cm × 1 cm faces
c) maximum when the battery is connected across 10 cm × 1/2 cm faces
d) same irrespective of the three faces
solution:The correct solution is a) maximum when the battery is connected across 1 cm × 1/2 cm faces
Two cells of emf’s approximately 5V and 10V are to be accurately compared using a potentiometer of length 400 cm.
a) the battery that runs the potentiometer should have voltage of 8V
b) the battery of potentiometer can have a voltage of 15V and R adjusted so that the potential drop across the wire slightly exceeds 10V
c) the first portion of 50 cm of wire itself should have a potential drop of 10V
d) potentiometer is usually used for comparing resistances and not voltages
Solution: The correct solution is b) the potentiometer's battery can be set to 15V and R adjusted so that the potential drop across the wire is a little higher than 10V.
A resistance R is to be measured using a meter bridge. Student chooses the standard resistance S to be 100Ω. He finds the null point at l1 = 2.9 cm . He is told to attempt to improve the accuracy. Which of the following is a useful way?
a) he should measure l1 more accurately
b) he should change S to 1000 Ω and repeat the experiment
c) he should change S to 3 Ω and repeat the experiment
d) he should give up hope of a more accurate measurement with a meter bridge
solution:The correct answer is c) he should change S to 3 Ω and repeat the experiment
Two batteries of emf ε1 and ε2 and internal resistances r1 and r2 respectively are connected in parallel as shown in the figure.a) the equivalent emf εeq of the two cells is between ε1 and ε2 that is ε1 < εeq < ε2
b) the equivalent emf εeq is smaller than ε1
c) the εeq is given by εeq = ε1 + ε2 always
d) εeq is independent of internal resistances r1 and r2
solution: The correct answer is a) the equivalent emf εeq of the two cells is between ε1 and ε2 that is ε1 < εeq < ε2
Consider a current-carrying wire in the shape of a circle. Note that as the current progresses along the wire, the direction of j changes in an exact manner, while the current I remain unaffected. The agent that is essentially responsible for is
a) source of emf
b) electric field produced by charges accumulated on the surface of wire
c) the charges just behind a given segment of wire which push them just the right way by repulsion
d) the charges ahead
solution: The correct answer is b) electric field produced by charges accumulated on the surface of wire
Write a test to differentiate between pentan-2-one and pentan-3-one.
Solution: One can differentiate between pentan-2-one and pentan-3-one by iodoform test. Pentan-2-one have a –CO-CH3 group and therefore forms a yellow precipitate of Iodoform. Pentan-2-one gives a...
Why is there a large difference in the boiling points of butanal and butane-1-ol?
Solution: Butanal has no intermolecular hydrogen bonding but butan-1-ol has intermolecular hydrogen bonding. This bonding in butan-1-ol makes it more stable at a higher temperature than butanal.
Which of the following is the correct representation for intermediate of nucleophilic addition reaction to the given carbonyl compound (A) :
Solution: Option (A) and (B) are the answers. Reason:
Benzophenone can be obtained by ____________. (i) Benzoyl chloride + Benzene + AlCl3 (ii) Benzoyl chloride + Diphenyl cadmium (iii) Benzoyl chloride + Phenyl magnesium chloride (iv) Benzene + Carbon monoxide + ZnCl2
Solution: Option (i) and (ii) are the answers Reason: Benzophenone can be obtained by the Friedel-Craft acylation reaction. The reaction is shown as
Through which of the number of the following reactions of carbon atoms can be increased in the chain? (i) Grignard reaction (ii) Cannizaro’s reaction (iii) Aldol condensation (iv) HVZ reaction
Solution: Option (i) and (iii) are the answers. Reason: Grigned reaction and aldol condensation is used to increase the number of carbon attom in the chain as follows:
Write Lewis dot symbols for atoms of the following elements :
a) Mg
b) Na
Solution: Only two valence electrons exist in the magnesium atom. As a result, the Lewis dot symbols for Mg are as follows: Only one valence electron exists in the sodium atom. As a...
Which of the following conversions can be carried out by Clemmensen Reduction? (i) Benzaldehyde into benzyl alcohol (ii) Cyclohexanone into cyclohexane (iii) Benzoyl chloride into benzaldehyde (iv) Benzophenone into diphenylmethane
Solution: Option (ii) and (iv) are the answers. Reason: The carbonyl group of aldehydes and ketones is reduced to CH2 group on treatment with zinc amalgam and concentrated hydrochloric acid...
Explain the formation of a chemical bond.
Answer: "A chemical bond is an attractive force that holds a chemical species' constituents together." For chemical bond formation, many theories have been proposed, including valence shell electron...
Treatment of compound with NaOH solution yields(i) Phenol (ii) Sodium phenoxide (iii) Sodium benzoate (iv) Benzophenone
Solution: Option (ii) and (iii) are the answers. Reason: Treatment of compound with NaOH yields sodium phenoxide and sodium by means of nucleophilic substitution reaction as follows
13. Which of the following compounds do not undergo aldol condensation?
Solution: Option (ii) and (iv) are the answers. reason: Aldehydes and ketones and having at least one alpha-hydrogen undergo a reaction in the presence of dilute alkali as catalyst to beta-hydroxy...
In Clemmensen Reduction carbonyl compound is treated with _____________. (i) Zinc amalgam + HCl (ii) Sodium amalgam + HCl (iii) Zinc amalgam + nitric acid (iv) Sodium amalgam + HNO3
Solution: Option (i) is the answer. Reason: From the above reaction carbonyl group is treated with Zn−Hg(Zinc Amalgum) and HCl
Which of the following compounds will give butanone on oxidation with alkaline KMnO4 solution? (i) Butan-1-ol (ii) Butan-2-ol (iii) Both of these (iv) None of these
Solution: Option (ii) is the answer.
Which is the most suitable reagent for the following conversion?(i) Tollen’s reagent (ii) Benzoyl peroxide (iii) I2 and NaOH solution (iv) Sn and NaOH solution
Solution: Option (iii) is the answer. Reason: This reaction is called as lodoform reaction.
Compound A and C in the following reaction are :_____________
Solution: Option (ii) is the answer. Reason:
Structure of ‘A’ and type of isomerism in the above reaction are respectively. (i) Prop–1–en–2–ol, metamerism (ii) Prop-1-en-1-ol, tautomerism (iii) Prop-2-en-2-ol, geometrical isomerism (iv) Prop-1-en-2-ol, tautomerism
Solution: Option (iv) is the answer. reason: Structure of A and the type of isomerism in the above reaction are Prop-1-en-2-ol, tautomerism respectively. Enol form tautomerises into keto...
Which product is formed when the compoundis treated with concentrated aqueous KOH solution?
Solution: Option (ii) is the answer. Reason: Benzaldhyde C6H5CHO on treatment with KOH yields the corresponding alcohol and acid. In this reaction, there is no alpha hydrogen atom present in...
Cannizaro’s reaction is not given by _____________.
Solution: Option (iv) is the answer. Reason: CH3CHO will not give Cannizzaro’s reaction because it contains a-hydrogen while other three compounds have no a-hydrogen. Hence, they will give...
The reagent which does not react with both, acetone and benzaldehyde. (i) Sodium hydrogen sulphite (ii) Phenyl hydrazine (iii) Fehling’s solution (iv) Grignard reagent
Solution: Option (iii) is the answer. Reason: Aromatic aldehydes and ketones does not respond to Fehling's test. Sodium hydrogen sulphite,phenyl hydrazine, grignard reaction are common for carbonyl...
Compound can be prepared by the reaction of _____________.
Solution: Option (ii) is the answer. Reason:
The correct order of increasing acidic strength is _____________. (i) Phenol < Ethanol < Chloroacetic acid < Acetic acid (ii) Ethanol < Phenol < Chloroacetic acid < Acetic acid (iii) Ethanol < Phenol < Acetic acid < Chloroacetic acid (iv) Chloroacetic acid < Acetic acid < Phenol < Ethanol
Solution: Option (iii) is the answer. Reason: The correct order of increasing acidic strength is Ethanol < Phenol < Acetic acid < Chloroacetic acid. Phenol is more acidic than ethanol...
Which of the following compounds is most reactive towards nucleophilic addition reactions?
Solution: Option (i) is the answer.
Addition of water to alkynes occurs in acidic medium and the presence of Hg2+ ions as a catalyst. Which of the following products will be formed on addition of water to but-1-one under these conditions.
Solution: Option (ii) is the answer. Reason: Addition of water to but-1-yne in the presence of H2SO4 and HgSO4 gives 2-butaone. The addition takes place by markovnikoff's rule....
Arrange the following compounds in increasing order of dipole moment. CH3CH2CH3, CH3CH2NH2, CH3CH2OH
Solution: CH3CH2CH3 < CH3CH2NH2 < CH3CH2OH The dipole moment of CH3CH2OH is greater than that of CH3CH2NH2. CH3CH2CH3 has the least dipole moment among the three given compounds because it is...
Predict the product of the reaction of aniline with bromine in a non-polar solvent such as CS2.
Solution: The products formed in the reaction of aniline with bromine in a non-polar solvent such as CS2 are 4-Bromoaniline and 2-Bromoaniline where 4-Bromoaniline is the major product.
Under what reaction conditions (acidic/basic), the coupling reaction of aryldiazonium chloride with aniline is carried out?
Solution: This reaction is carried out in a mild basic medium. This is an electrophilic substitution reaction. Aryldiazonium chloride reacts with aniline to form a yellow dye of p-Aminoazobenzene.
Explain why MeNH2 is a stronger base than MeOH?
Solution: MeNH2 is a stronger base than MeOH because of the lower electronegativity and the presence of the lone pair of electrons on the nitrogen atom in MeNH2.
Why does the acetylation of —NH2 group of aniline reduce its activating effect?
Solution: The acetylation of —NH2 group of aniline reduces its activating effect because the lone pair of electrons on the nitrogen of acetanilide interacts with oxygen atom due to resonance.
Why is benzene diazonium chloride not stored and is used immediately after its preparation?
Solution: At high temperatures, benzene diazonium chloride is highly soluble in water, and at low temperatures, it is a very stable compound in water. Because it is unstable, it should be used as...
What is Hinsberg reagent?
Solution: Hinsberg's reagent is benzene sulphonyl chloride, also known as $C_6H_5SOCl$. To distinguish between primary, secondary, and tertiary amines, Hinsberg's reagent is used.
What is the best reagent to convert nitrile to primary amine?
Solution: LiAlH4 and Sodium/Alcohol are the best reagents for converting nitrile to primary amine. The nitriles can be converted into a corresponding primary amine through reduction.
What is the product when C6H5CH2NH2 reacts with HNO2?
Solution: The main product is $C_6H_5CH_2OH$ When $C_6H_5CH_2NH_2$ reacts with HNO2, it produces an unstable diazonium salt, which is then converted to alcohol. When $C_6H_5CH_2NH_2$ reacts with...
Why is NH2 group of aniline acetylated before carrying out nitration?
Solution: The NH2 group of aniline is acetylated before nitration to control the nitration reaction and the formation of tarry oxidation products and nitro derivatives. P-nitroaniline is the main...
What is the role of HNO3 in the nitrating mixture used for nitration of benzene?
Solution: The nitration of organic compounds is done with a nitration mixture, which is a 1:1 solution of HNO3 and H2SO4. In the nitration of benzene, it acts as a base and provides electrophile.
Which of the following reactions belong to electrophilic aromatic substitution?
(i) Bromination of acetanilide
(ii) Coupling reaction of aryldiazonium salts
(iii) Diazotisation of aniline
(iv) Acylation of aniline
Solution: Option (i) and (ii) are the answers. Reason:...
Refer to the graphs below and match the following:
Graph Characteristics a) i) has v > 0 and a < 0 throughout b) ii) has x > 0 throughout and has a point with v = 0 and a point with a = 0 c) iii) has a point with zero displacement for t...
Under which of the following reaction conditions, aniline gives p-nitro derivative as the major product?
(i) Acetyl chloride/pyridine followed by reaction with conc. H2SO4 + conc. HNO3
(ii) Acetic anhydride/pyridine followed by conc. H2SO4 + conc. HNO3
(iii) Dil. HCl followed by reaction with conc. H2SO4 + conc. HNO3
(iv) Reaction with conc. HNO3 + conc.H2SO4
Solution: Option (i) and (ii) are the answers. Reason: In addition to the nitro derivatives, direct nitration of aniline produces tarry oxidation products. Furthermore, in a strongly acidic...
Which of the following reactions are correct?
Solution: Option (i) and (iii) are the answers. Reason:
Which of the following amines can be prepared by Gabriel synthesis.
(i) Isobutyl amine
(ii) 2-Phenylethylamine
(iii) N-methyl benzylamine
(iv) Aniline
Solution: Option (i) and (ii) are the answers. Reason: Gabriel synthesis is used for the preparation of primary amines. Phthalimide on treatment with ethanolic potassium hydroxide forms potassium...
Arenium ion involved in the bromination of aniline is __________.
Solution: Option (i), (ii) and (iii) are the answers. Reason: Arenium ion involved in the bromination of aniline are as follows:
The product of the following reaction is __________.
Solution: Option (A) and (B) is the answer. Reason:
The reagents that can be used to convert benzene diazonium chloride to benzene are __________.
(i) 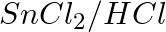
(ii) 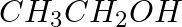
(iii) 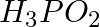
(iv) 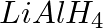
Solution: Option (ii) and (iii) are the answers. Reason: Hypophosphorous acid (phosphinic acid) and ethanol, for example, reduce diazonium salts to arenes, which are then oxidised to phosphorous...
Which of the following species are involved in the carbylamine test?
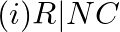
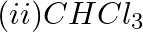
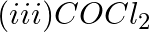
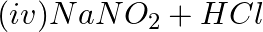
Solution: Option (i) and (ii) are the answers. Reason: Only RNC and CHCl3 are involved in carbylamine reaction.
Reduction of nitrobenzene by which of the following reagent gives aniline?
(i) Sn/HCl
(ii) Fe/HCl
(iii) H2-Pd
(iv) Sn/NH4OH
Solution: Option (i), (ii), and (iii) are the answers. Reason: They are reducing agents.
Which of the following cannot be prepared by Sandmeyer’s reaction?
(i) Chlorobenzene
(ii) Bromobenzene
(iii) Iodobenzene
(iv) Fluorobenzene
Solution: Option (iii) and (iv) are the answers. Reason: Sandmeyer's reaction is used for the preparation of chlorobenzene and bromobenzene.
Which of the following methods of preparation of amines will not give the same number of carbon atoms in the chain of amines as in the reactant?
(i) The reaction of nitrite with LiAlH4.
(ii) The reaction of the amide with LiAlH4 followed by treatment with water.
(iii) Heating alkyl halide with potassium salt of phthalimide followed by hydrolysis.
(iv) Treatment of amide with bromine in the aqueous solution of sodium hydroxide.
Solution: Option (iv) is the answer. Reason: In Hoffmann Bromide degradation as the word, suggest, the amide is reduced to an amine with 1- carbonless, so this is the method in which we don't get...
Which of the following should be most volatile?
Solution: Option (ii) is the answer. Reason: The order of boiling points of isomeric amines is 1 amine > 2 amines > 3 amines. 3 amines have no intermolecular association because there are no H...
Among the following amines, the strongest Brönsted base is __________.
Solution; Option (iv) is the answer. Reason: Option (iv) is the strongest Bronsted base as there is no delocalization of lone pair of electron of the atom which is not possible in aniline and in...
The correct decreasing order of basic strength of the following species is _______. H2O, NH3, OH–, NH2– (i) NH2– > OH – > NH3 > H2O (ii) OH– > NH2– > H2O > NH3 (iii) NH3 > H2O > NH2– > OH– (iv) H2O > NH3> OH– > NH2–
Solution: Option (i) is the answer. Reason: NH3 is more basic than H2O, therefore NH2− (Conjugate base of weak acid NH3) is a stronger base than OH−.
Among the following amines, the strongest Brönsted base is __________.
Solution; Option (iv) is the answer. Reason: Option(iv)is the strongest Bronsted base as there is no delocalisation of lone pair of electron of N atom which is not possible in aniline and in...
Which of the following compounds is the weakest Brönsted base?
Solution: Option (iii) is the answer. Reason: A Bronsted Lowry base is a proton acceptor or hydrogen ion acceptor. Amines have a stronger tendency to accept protons and are strong Bronsted bases....
Which of the following compound will not undergo an azo coupling reaction with benzene diazonium chloride.
(i) Aniline
(ii) Phenol
(iii) Anisole
(iv) Nitrobenzene
Solution: Option (iv) is the answer. Reason: Diazonium cation is a weak electrophile and hence it reacts with electron-rich compounds containing electron-donating groups such as −OH, -$NH_2$ and...
The best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is
(i) Hoffmann Bromamide reaction
(ii) Gabriel phthalimide synthesis
(iii) Sandmeyer reaction
(iv) Reaction with 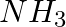
Solution: Option (ii) is the answer. Reason: Best method for preparing primary aminos form alkyl halides without changing the number of carbon atoms in the chain is Gabriel synthesis. Because this...
The reaction Ar + N2Cl– → (Cu/HCl)– ArCl + N2 + CuCl is named as _________.
(i) Sandmeyer reaction
(ii) Gatterman reaction
(iii) Claisen reaction
(iv) Carbylamine reaction
Solution: Option (ii) is the answer. Reason: Diazonium salts in the presence of copper powder and halogen acid give aryl halide. Gattermann reaction is a variation of Sandmeyer reaction in which...
Acid anhydrides on reaction with primary amines give ____________.
(i) amide
(ii) imide
(iii) secondary amine
(iv) imine
Solution: Option (i) is the answer Reason: When acid anhydrides react with primary amines, they produce amide. The H atom of the amino group is replaced with an acyl group in this nucleophilic...
The most reactive amine towards dilute hydrochloric acid is ___________.
Solution: Option (ii) is the answer. Reason: The reactivity of amines is proportional to their basicity. If the R group is, the order of basicity is secondary amine ...
Reduction of aromatic nitro compounds using Fe and HCl gives __________.
(i) aromatic oxime
(ii) aromatic hydrocarbon
(iii) aromatic primary amine
(iv) aromatic amide
Solution: Option (iii) is the answer. Reason: Reduction of nitro aryl compounds in presence of Fe and HCl gives aromatic primary amines.
In the nitration of benzene using a mixture of conc.  and conc.
and conc. 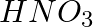 ,the species which initiates the reaction is __________.
,the species which initiates the reaction is __________.
(i) 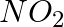
(ii) 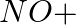
(iii) 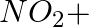
(iv) 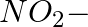
Solution: Option (iii) is the answer. Reason:
The gas evolved when methylamine reacts with nitrous acid is __________.
(i) 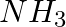 (ii)
(ii) 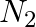 (iii)
(iii) 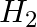 (iv)
(iv) 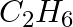
Solution: Option (ii) is the answer. Reason:
Methylamine reacts with HNO2 to form _________.
(i) 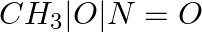 (ii)
(ii) 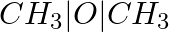 (iii)
(iii) 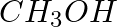 (iv)
(iv) 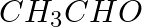
Solution: Option (iii) is the answer. Reason:
The correct increasing order of basic strength for the following compounds is _________.
(i) II < III < I
(ii) III < I < II
(iii) III < II < I
(iv) II < I < III
Solution: Option (iv) is the answer. Reason: Electron donating: group increases the basicity while electron-withdrawing group decreases the basicity of...
Hoffmann Bromamide Degradation reaction is shown by __________.
(i) 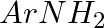
(ii) 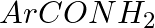
(iii) 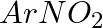
(iv) 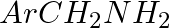
Solution: Option (ii) is the answer. Reason: Where the aryl amide is converted to arylamine in the presence of $Br_2$ and $NaOH$ .
The best reagent for converting 2–phenylpropanamide into 2-phenylpropanolamine is _____.
(i) excess H2 (ii) Br2 in aqueous NaOH (iii) iodine in the presence of red phosphorus (iv) LiAlH4 in ether
Solution: Option (iv) is the answer. Reason:
Amongst the given set of reactants, the most appropriate for preparing 2° amine is _____.
(i) 2° R—Br + NH3
(ii) 2° R—Br + NaCN followed by 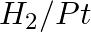
(iii) 1° R—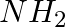 + RCHO followed by %H_2/Pt
+ RCHO followed by %H_2/Pt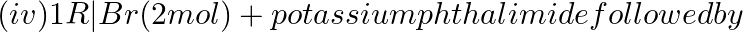 H_3O+$/heat
H_3O+$/heat
Solution: Option (iii) is the answer. Reason:
The source of nitrogen in Gabriel synthesis of amines is _____________.
(i) Sodium azide, NaN3
(ii) Sodium nitrite, NaNO2
(iii) Potassium cyanide, KCN
(iv) Potassium phthalimide
Solution: Option (iv) is the answer. Reason: Gabriel synthesis :The reaction is given to the image.Source of nitrogen atom is Gabriel synthesis is Potassium phthalamide.
To prepare a 1° amine from an alkyl halide with simultaneous addition of one 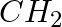 group in the carbon chain, the reagent used as a source of nitrogen is ___________.
group in the carbon chain, the reagent used as a source of nitrogen is ___________.
(i) Sodium amide, NaNH2
(ii) Sodium azide, NaN3
(iii) Potassium cyanide, KCN
(iv) Potassium phthalimide
Solution: Option (iii) is the answer. Reason:
6. Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine? (i) H2 (excess)/Pt (ii) LiAlH4 in ether (iii) Fe and HCl (iv) Sn and HCl
Solution: Option (ii) is the answer. Reason: LiAlH4/ether reduces aryl nitro compounds to azo compounds 2C6H5NO2→LiAIH4C6H5N=N-C6H5
5. Benzylamine may be alkylated as shown in the following equation : C6H5CH2NH2 + R—X → C6H5CH2NHR Which of the following alkyl halides is best suited for this reaction through SN1 mechanism? (i) CH3Br (ii) C6H5Br (iii) C6H5CH2Br (iv) C2H5 Br
Solution: Option (iii) is the answer. Reason: C6H5CH2Br is best suited for this reaction through SN1 mechanism as the carbocation (C6H5CH2) formed is resonance...
Which of the following is the weakest Brönsted base?
Solution: Option (A) is the answer. Reason: Aniline is the weakest Bronsted base due to delocalization of lone pair of electron...
Amongst the following, the strongest base in aqueous medium is ____________.
(i) 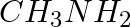
(ii) 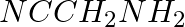
(iii) 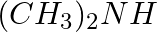
(iv) 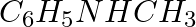
Solution: Option (iii) is the answer. Reason: Due to the electron releasing nature of the alkyl group, it (R) pushes electrons towards nitrogen and thus makes the uncharged electrons pair available...
The correct IUPAC name for 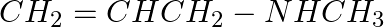 is (i) Allylmethylamine (ii) 2-amino-4-pentene (iii) 4-aminopent-1-ene (iv) N-methylprop-2-en-1-amine
is (i) Allylmethylamine (ii) 2-amino-4-pentene (iii) 4-aminopent-1-ene (iv) N-methylprop-2-en-1-amine
Solution: Option (iv) is the answer. Reason: $CH_2=CHCH_2-NHCH_3$ N−methylprop−2−en−1−amine.
Which of the following is a 3° amine?(i) 1-methylcyclohexylamine (ii) Triethylamine (iii) tert-butylamine (iv) N-methyl aniline
Solution: Option (ii) is the answer. Reason: Triethylamine is a 3° amine because of amonia in which each hydrogen atom is substituted by an methyl group.
Show the 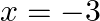 is a solution of
is a solution of 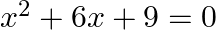
The given equation is $x^{2}+6 x+9=0$ Putting $x=-3$ in the given equation, we get $L H S=(-3)^{2}+6 \times(-3)+9=9-18+9=0=R H S$ $\therefore x=-3$ is a solution of the given equation.
Find dy/dx in each of the following: 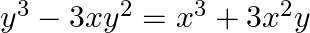
differentiating the equation on both sides with respect to x, we get,
A train covers a distance of 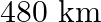 at a uniform speed. If the speed had been
at a uniform speed. If the speed had been 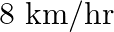 less then it would have taken 3 hours more to cover the same distance. Find the usual speed of the train.
less then it would have taken 3 hours more to cover the same distance. Find the usual speed of the train.
Let the usual speed of the train be $x \mathrm{~km} / \mathrm{h}$. $\therefore$ Reduced speed of the train $=(x-8) \mathrm{km} / \mathrm{h}$ Total distance to be covered $=480 \mathrm{~km}$ Time...
![Rendered by QuickLaTeX.com \[\text { If } A=\left[\begin{array}{cc} 2 & -1 \\ -1 & 2 \end{array}\right], \text { such that } A^{2}-4 A+3 I=0 \text {, then } A^{-1}=\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7b894a5fdb230f303ed2c3138014aee7_l3.png)
(A) ![Rendered by QuickLaTeX.com \frac{-1}{3}\left[\begin{array}{cc}2 & 1 \\ 1 & 2\end{array}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-3de2c9c89535b15d716c4004abf378ac_l3.png) (B)
(B) ![Rendered by QuickLaTeX.com \frac{-1}{3}\left[\begin{array}{cc}2 & -1 \\ -1 & 2\end{array}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-2c0ef8c38089f5461a5c3cae75564dbc_l3.png) (C)
(C) ![Rendered by QuickLaTeX.com \frac{1}{3}\left[\begin{array}{cc}-2 & -1 \\ 1 & -2\end{array}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-b29c7040a237cfd2f9f8fbf9de6861f6_l3.png) (D)
(D) ![Rendered by QuickLaTeX.com \frac{1}{3}\left[\begin{array}{ll}2 & 1 \\ 1 & 2\end{array}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ee435aff6cf5f9f19b76d6e3447d2f91_l3.png)
![Rendered by QuickLaTeX.com \[\text { If } A=\left[\begin{array}{cc} 2 & -1 \\ -1 & 2 \end{array}\right], \text { such that } A^{2}-4 A+3 I=0 \text {, then } A^{-1}=\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7b894a5fdb230f303ed2c3138014aee7_l3.png)
The correct option is option (D) $\therefore \mathrm{A}^{2}=\mathrm{A} \cdot \mathrm{A}=\left[\begin{array}{cc}2 & -1 \\ -1 & 2\end{array}\right] \cdot\left[\begin{array}{cc}2 & -1 \\ -1...
A teacher on attempting to arrange the students for mass drill in the form of solid square found that 24 students were left. When he increased the size of the square by one student, he found that he was short of 25 students. Find the number of students.
Let there be $x$ rows. Then, the number of students in each row will also be $x$. $\therefore$ Total number of students $=\left(x^{2}+24\right)$ According to the question: $\begin{array}{l}...
The sum of a natural number and its square is 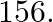 Find the number.
Find the number.
Let the required natural number be $x$. According to the given condition, $x+x^{2}=156$ $\Rightarrow x^{2}+x-156=0$ $\Rightarrow x^{2}+13 x-12 x-156=0$ $\Rightarrow x(x+13)-12(x+13)=0$...
A manufacturer produces two Models of bikes – Model  and Model
and Model  . Model
. Model  takes 6 man-hours to make per unit, while Model Y takes 10 man-hours per unit. There is a total of 450 man-hour available per week. Handling and Marketing costs are Rs 2000 and Rs 1000 per unit for Models
takes 6 man-hours to make per unit, while Model Y takes 10 man-hours per unit. There is a total of 450 man-hour available per week. Handling and Marketing costs are Rs 2000 and Rs 1000 per unit for Models  and
and 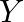 respectively. The total funds available for these purposes are Rs 80,000 per week. Profits per unit for Models
respectively. The total funds available for these purposes are Rs 80,000 per week. Profits per unit for Models 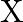 and
and 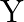 are Rs 1000 and Rs 500 , respectively. How many bikes of each model should the manufacturer produce so as to yield a maximum profit? Find the maximum profit.
are Rs 1000 and Rs 500 , respectively. How many bikes of each model should the manufacturer produce so as to yield a maximum profit? Find the maximum profit.
Solution: Let us take $\mathrm{x}$ an $\mathrm{y}$ to be the no. of models of bike produced by the manufacturer. From the question we have, Model $x$ takes 6 man-hours to make per unit Model $y$...
Maximize 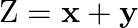 subject to
subject to 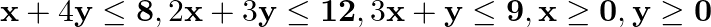 .
.
Solution: It is given that: $Z=x+y$ subject to constraints, $x+4 y \leq 8$ $2 x+3 y \leq 12,3 x+y \leq 9, x \geq 0, y \geq 0$ Now construct a constrain table for the above, we have Here, it can be...
Refer to Exercise 15. Determine the maximum distance that the man can travel.
Solution: According to the solution of exercise 15, we have Maximize $Z=x+y$, subject to the constraints $2 x+3 y \leq 120 \ldots$ (i) $8 x+5 y \leq 400 \ldots$ (ii) $x \geq 0, y \geq 0$ Let's...
Refer to Exercise 14. How many sweaters of each type should the company make in a day to get a maximum profit? What is the maximum profit?
Solution: According to the solution of exercise 14, we have Maximize $Z=200 x+120 y$ subject to constrains $\begin{array}{l} 3 x+y \leq 600 \ldots \text { (i) } \\ x+y \leq 300 \ldots \text { (ii) }...
Refer to Exercise 13. Solve the linear programming problem and determine the maximum profit to the manufacturer.
Solution: According to the solution of exercise 13, we have The objective function for maximum profit $\mathrm{Z}=100 \mathrm{x}+170 \mathrm{y}$ Subject to constraints, $x+4 y \leq 1800 \ldots(i)$...
Refer to Exercise 12. What will be the minimum cost?
Solution: According to the solution of exercise 12, we have The objective function for minimum cost is $\mathrm{Z}=400 \mathrm{x}+200 \mathrm{y}$ Subject to the constrains; $5 x+2 y \geq 30 \ldots...
Refer to Exercise 11. How many of circuits of Type A and of Type B, should be produced by the manufacturer so as to maximize his profit? Determine the maximum profit.
Solution: According to the solution of exercise 11, we have Maximize $Z=50 x+60 y$ subject to the constraints $20 x+10 y \leq 2002 x+y \leq 20 \ldots$ (i) $10 x+20 y \leq 120 x+2 y \leq 12 \ldots$...
A man rides his motorcycle at the speed of 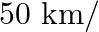 hour. He has to spend Rs 2 per km on petrol. If he rides it at a faster speed of
hour. He has to spend Rs 2 per km on petrol. If he rides it at a faster speed of 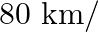 hour, the petrol cost increases to Rs 3 per km. He has at most Rs 120 to spend on petrol and one hour’s time. He wishes to find the maximum distance that he can travel. Express this problem as a linear programming problem.
hour, the petrol cost increases to Rs 3 per km. He has at most Rs 120 to spend on petrol and one hour’s time. He wishes to find the maximum distance that he can travel. Express this problem as a linear programming problem.
Solution: Suppose the man covers $\mathrm{x} \mathrm{km}$ on his motorcycle at the speed of $50 \mathrm{~km} / \mathrm{hr}$ and covers $\mathrm{y} \mathrm{km}$ at the speed of $50 \mathrm{~km} /...
A company manufactures two types of sweaters: type A and type B. It costs Rs 360 to make a type A sweater and Rs 120 to make a tvpe B sweater. The companv can make at most 300 sweaters and spend at most Rs 72000 a day. The number of sweaters of type B cannot exceed the number of sweaters of type A by more than 100 . The company makes a profit of Rs 200 for each sweater of type A and Rs 120 for every sweater of type B. Formulate this problem as a LPP to maximize the profit to the company.
Solution: Suppose $\mathrm{x}$ and $\mathrm{y}$ to be the number of sweaters of type $\mathrm{A}$ and type $\mathrm{B}$ respectively. The following constraints are: $360 x+120 y \leq 72000...
A company manufactures two types of screws A and B. All the screws have to pass through a threading machine and a slotting machine. A box of Type A screws requires 2 minutes on the threading machine and 3 minutes on the slotting machine. A box of type  screws requires 8 minutes of threading on the threading machine and 2 minutes on the slotting machine. In a week, each machine is available for 60 hours. On selling these screws, the company gets a profit of Rs 100 per box on type A screws and Rs 170 per box on type B screws. Formulate this problem as a LPP given that the objective is to maximize profit.
screws requires 8 minutes of threading on the threading machine and 2 minutes on the slotting machine. In a week, each machine is available for 60 hours. On selling these screws, the company gets a profit of Rs 100 per box on type A screws and Rs 170 per box on type B screws. Formulate this problem as a LPP given that the objective is to maximize profit.
Solution: Suppose that the company manufactures $\mathrm{x}$ boxes of type A screws and $y$ boxes of type B screws. The below table is constructed from the information provided:...
A firm has to transport 1200 packages using large vans which can carry 200 packages each and small vans which can take 80 packages each. The cost for engaging each large van is Rs 400 and each small van is Rs 200 . Not more than Rs 3000 is to be spent on the iob and the number of large vans cannot exceed the number of small vans. Formulate this problem as a LPP given that the objective is to minimize cost.
Solution: Suppose $\mathrm{x}$ and $\mathrm{y}$ to be the number of large and small vans respectively. The below constrains table is constructed from the information provided:...
A manufacturer of electronic circuits has a stock of 200 resistors, 120 transistors and 150 capacitors and is required to produce two types of circuits A and B. Type A requires 20 resistors, 10 transistors and 10 capacitors. Type B requires 10 resistors, 20 transistors and 30 capacitors. If the profit on type A circuit is Rs 50 and that on type B circuit is Rs 60 , formulate this problem as a LPP so that the manufacturer can maximize his profit.
Solution: Suppose $\mathrm{x}$ units of type A and $y$ units of type $\mathrm{B}$ electric circuits be produced by the manufacturer. The table is constructed from the information provided:...
In Fig. 12.11, the feasible region (shaded) for a LPP is shown. Determine the maximum and minimum value of 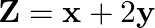
Solution: It is seen from the given figure, that the corner points are as follows: $\mathrm{R}(7 / 2,3 / 4), \mathrm{Q}(3 / 2,15 / 4), \mathrm{P}(3 / 13,24 / 13)$ and $\mathrm{S}(18 / 7,2 / 7)$ On...
The feasible region for a LPP is shown in Fig. 12.10. Evaluate 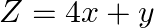 at each of the corner points of this region. Find the minimum value of
at each of the corner points of this region. Find the minimum value of 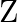 , if it exists.
, if it exists.
Solution: It is given that: $Z=4 x+y$ In the figure given, $\mathrm{ABC}$ is the feasible region which is open unbounded. Here, we get $x+y=3\dots \dots(i)$ and $\quad x+2 y=4 \quad \ldots$ (ii) On...
Refer to Exercise 7 above. Find the maximum value of 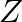 .
.
Solution: It is clearly seen that the evaluating table for the value of $Z$, the maximum value of $Z$ is 47 at $(3,2)$
The feasible region for a LPP is shown in Fig. 12.9. Find the minimum value of 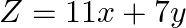 .
.
Solution: It is seen from the given figure, that the feasible region is $\mathrm{ABCA}$. Corner points are $\mathrm{C}(0,3), \mathrm{B}(0,5)$ and for A, we have to solve equations $x+3 y=9$ and...
Feasible region (shaded) for a LPP is shown in Fig. 12.8. Maximize 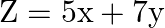 .
.
Solution: It is given that: $\mathrm{Z}=5 \mathrm{x}+7 \mathrm{y}$ and feasible region $\mathrm{OABC}$. Corner points of the feasible region are $\mathrm{O}(0,0), \mathrm{A}(7,0), \mathrm{B}(3,4)$...
Determine the maximum value of 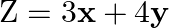 if the feasible region (shaded) for a LPP is shown in Fig. 12.7.
if the feasible region (shaded) for a LPP is shown in Fig. 12.7.
Solution: OAED is the feasible region, as shown in the figure At $A, y=0$ in eq. $2 x+y=104$ we obtain, $\mathrm{x}=52$ This is a corner point $A=(52,0)$ At $D, x=0$ in eq. $x+2 y=76$ we obtain,...
Minimize 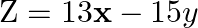 subject to the constraints:
subject to the constraints: 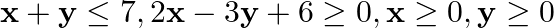 .
.
Solution: It is given that: $\mathrm{Z}=13 \mathrm{x}-15 \mathrm{y}$ and the constraints $\mathrm{x}+\mathrm{y} \leq 7$, $2 x-3 y+6 \geq 0, x \geq 0, y \geq 0$ Taking $x+y=7$, we have...
