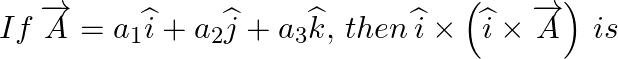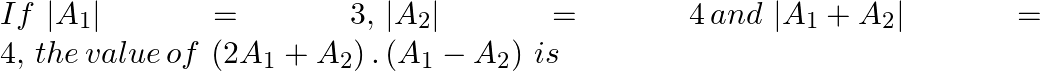The correct option is (A) 0 Since reactive impedance at resonance is zero and we know that, $\tan \phi=\frac{X_{L}-X_{C}}{Z}$ but $\mathrm{X}_{\mathrm{L}}-\mathrm{X}_{\mathrm{C}}=0$ therefore...
The phase difference between the current and voltage at resonance is
The scalar product of 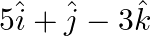 and
and 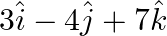 is (A) 10 (B)
is (A) 10 (B) 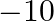 6 (C) 15 (D)
6 (C) 15 (D) 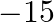
The scalar product of $5 \hat{i}+\hat{j}-3 \hat{k}$ and $3 \hat{i}-4 \hat{j}+7 \hat{k}$ is (A) 10 (B) $-10$ 6 (C) 15 (D) $-15$ Sol. Correct answer option is (B) $-10$ 6
Q80) Eukaryotic cells do not possess ________ . a. Ribosomes b. Mesosomes c. Fimbriae d. Mitochondria 1. Only b 2. b and c 3. a and b 4. only d
Correct option: 2 Explanation: Eukaryotic cells do not possess mesosomes and fimbriae.
Q79) Sexual reproduction is absent in the membranes of _________ . 1. Deuteromycetes 2. Phycomycetes 3. Basidiomycetes 4. Ascomycetes
Correct option: 1 Explanation: Sexual reproduction is absent in the membranes of deuteromycetes. Deuteromycetes are also known as fungi imperfecta. Asexual reproduction may take place through...
Q78) The enzymes needed for Krebs cycle are located in ______ . 1. Oxysomes of mitochondria 2. Matrix of mitochondria 3. Cytoplasm of cell 4. Outer membrane of mitochondria
Correct option: 2 Explanation: The enzymes needed for Krebs cycle are located in matrix of mitochondria. The Krebs cycle enzymes are membrane proteins found within the matrix of the mitochondria.
Q77) Damage to VI cranial nerve in human, may affect the movements of ________ . 1. Neck 2. Jaw 3. Tongue 4. Eye
Correct option: 4 Explanation: Damage to VI cranial nerve in human, affects the movements of eye. Sixth nerve palsy is a condition that affects the eye movement. The main function of VI cranial...
Q76) The size of genome of Methanococcus jannaschii is ______ . 1. 1830 kb 2. 1660 kb 3. 569 kb 4. 12,500 kb
Correct option: 2 Explanation: The size of genome of Methanococcus jannaschii is 1660kb. The total amount of DNA contained in one copy of a single complete genome is referred to as a genome.
Q75) In Morgan’s experiment on Drosophilia cross between yellow bodied, white eyed female with wild type male gives ______ % of parental gene combination in F2 generation. 1. 37% 2. 1.3% 3. 62.8% 4. 98.7%
Correct option: 4 Explanation: In Morgan’s experiment on Drosophilia cross between yellow bodied, white eyed female with wild type male gives 98.7 % of parental gene combination in F2...
Q74) A man working in a furnace room suffered from asphyxiation. What should be the main reason? 1. More O2 level in the furnace room. 2. CO poisoning due to high CO level in the furnace room. 3. More O2 as well as fumes in the furnace room. 4. Less O2 level in the furnace room.
Correct option: 2 Explanation: The man suffered from asphyxiation because of CO poisoning due to high CO level in the furnace room. Carbon monoxide is a harmful gas that combines with haemoglobin to...
Q73) In plant breeding, biofortification is a method ______ . 1. To increase the nutritional value of crop plants. 2. To make the crop plants disease resistant. 3. To improve the yield of the crop plant.
Correct option: 1 Explanation: In plant breeding, biofortification is a method to increase the nutritional value of crop plants. Biofortification is a way by which the nutrients of the plants are...
Q72) Genetic material present in prokaryotes _______ is 1. Nucleosome 2. Nucleus 3. Nucleolus 4. Nucleoid
Correct option: 4 Explanation: nucleoid is the genetic material present in prokaryotes. It is an irregular shaped region present in the prokaryotes.
Q71) Norman Borlaug developed semi-dwarf varieties of wheat in _____ . 1. Mexico 2. USA 3. Philippines 4. India
Correct option: 1 Explanation: the Nobel Laureate , Norman E. Borlang at International Centre for Wheat and Maize Improvement in Mexico developed semi-dwarf varieties of wheat
Q70) The number of phenotypic and genotypic individuals produced during a typical Mendelian monohybrid cross will be ______ and _____ respectively. 1. 2 and 2 2. 3 and 2 3. 2 and 3 4. 3 and 3
Correct option: 3 Explanation: The number of phenotypic and genotypic individuals produced during a typical Mendelian monohybrid cross will be 2 and 3 respectively.
Q69) Select the incorrect statement 1. Hisardale is an example of cross-breeding experiment. 2. Genetic mother in MOET technique serves for multiple ovulation. 3. Apis mellifera and Apis florea domesticated species of honey bee. 4. Layers management includes processes like culling and debeaking.
Correct option: 3 Explanation: Apis mellifera and Apis florea are not domesticated species of honey bee
Q68) What is the percentage of methane in biogas? 1. 15 – 45 2. 91 – 95 3. 85 – 90 4. 50 – 80
Correct option: 4 Explanation: the percentage of methane in biogas is 50 – 80%. This percentage of methane in biogas is determined by Orsat apparatus gas-chromatography etc. We can also say that it...
Q67) The vector phage lambda is commonly used for gene transfer in ____ . 1. Plant cell 2. Yeast 3. Bacteria 4. Insect
Correct option: 3 Explanation: The vector phage lambda is commonly used for gene transfer in bacteria. Lambda phage consists of virus particle including a head, tall and tail fibers.
Q66) The product of syngamy in angiosperms is ________ . 1. Egg 2. PEN 3. Oopshere 4. Oospore
Correct option: 4 Explanation: oospore is the product of syngamy in angiosperms. It is a thick walled sexual spores. In syngamy two nuclei from each of the pollen tubes go on to fertilize two other...
Q65) Majority of kidney stones are formed by _____ . 1. Uric acid 2. Urea 3. Calcium oxalate 4. Cystine
Correct option: 3 Explanation: Majority of kidney stones are formed by calcium oxalate. Kidney stones are solid masses that form in the kidney, due to increased levels of calcium, oxalate, cystine...
Q64) Spinal cord is enclosed in _____ of vertebral column. 1. Neural canal 2. Volkman’s canal 3. Inguinal canal 4. Central canal
Correct option: 1 Explanation: Spinal cord is enclosed in neural canal of vertebral column. Spinal cord is a long bundle of nerves and cells that stretch from the lower portion of the brain to the...
Q63) The number of carbon atoms per molecule of citric acid, oxaloacetic acid and pyruvic acid respectively are ______ . 1. 4, 6 and 3 2. 6, 4 and 3 3. 6, 3 and 2 4. 4, 4 and 3
Correct option: 2 Explanation: The number of carbon atoms per molecule of citric acid, oxaloacetic acid and pyruvic acid respectively are 6, 4 and 3. Pyruvic acid is a 3 carbon compound,...
Q62) In recombinant DNA technology after the bacteriophage infects a bacterial cell, plaques are formed by the _____ . 1. New colonies of bacterial cells 2. Infecting bacteriophages 3. Lysed bacterial cells 4. Virions
Correct option: 3 Explanation: In recombinant DNA technology after the bacteriophage infects a bacterial cell, plaques are formed by the lysed bacterial cells. Plaques are clear zones formed in a...
Q61) which phytochrome promotes seed germination in cereals by synthesizing amylase enzyme? 1. Gibberellin 2. Abscisic acid 3. Auxin 4. Cytokinin
Correct option: 1 Explanation: gibberellin promotes seed germination in cereals by synthesizing amylase enzyme. Gibberellins cause seed germination by breaking the dormancy of the seed and acts as a...
Q59) High aspartic acid. Low nitrogen and sugar content in maize variety makes it resistant to _____ . 1. Leaf curl 2. Black rot 3. Stripe rust 4. Stem border
Correct option: 4 Explanation: maize plant exhibits an example of development of pest resistant because of the biochemical characters. Low nitrogen, sugar and high aspartic acid impart the plants...
Q58) In the process of digestion, lipids present in oily and fried foods are digested and split into _____ and ____ . 1. Fatty acids and glycerol 2. Glucose and glycerol 3. Fatty acids and amino acids 4. Glucose and amino acids
Correct option: 1 Explanation: In the process of digestion, lipids present in oily and fried foods are digested and split into fatty acids and glycerol. The enzyme lipase breaks the fat into fatty...
Q57) Match the column I with column II and select the correct option. Column I Column II A. Tidal volume i) 2500-3000 ml B. Inspiratory reserve volume ii) 1000 ml C. Residual volume iii) 4500 ml D. Vital capacity iv) 500 ml 1. A- iii, B- iv, C-i, D-ii 2. A- ii, B- iii, C-iv, D-i 3. A- iv, B- i, C-ii, D-iii 4. A- iv, B- ii, C-iii, D-i
Correct option: 3 Explanation: Normal tidal volume in human beings is 500 ml. Inspiratory reserve volume is 2500 – 3000 ml. It is defined as the amount of air that can be forcibly inhaled after a...
Q56) Which one of the following heavy metals in water is responsible to cause haemolysis, diarrhoea, abdominal and chest pain in humans? 1. Selenium 2. Arsenic 3. Mercury 4. Lead
Correct option: 3 Explanation: chronic mercury poisoning can cause diarrhea, chest pain, abdominal pain, nausea, hemolysis, vomiting, and many more symptoms have been seen.
Q55) Photosynthetic organisms which lack chlorophyll-a are _____ . 1. Vascular plants 2. Algae 3. Photosynthetic bacteria 4. Bryophytes
Correct option: 3 Explanation: Photosynthetic bacteria donot have chlorophyll to absorb light. Instead they have bacteriochlorophyll, which can absorb short wavelengths of light than the...
Q54) Grafting is not possible in monocots because they do not possess ____ . 1. Branches 2. Buds 3. Apical meristem 4. Cambium
Correct option: 4 Explanation: monocots cannot be grafted as they donot have cambium tissue. The dicot plants have cambium tissue which is a meristematic tissue found in the vascular bundles of the...
Q53) Select the correct statement with reference to AIDS. 1. It spreads through contact with HIV infected person. 2. It is easily curable. 3. Patients called non-progressors develop AIDS very slowly or never at all. 4. It is transmitted through insect bite.
Correct option: 3 Explanation: AIDS also known as human immunodeficiency syndrome. It interferes with the capacity of the body to fight against the infections. It is not easily curable and not...
Q52) Inflammation of alveoli, consolidation in lungs, are characteristics of ____ disease. 1. Malaria 2. Amoebiasis 3. Filariasis 4. Pneumonia
Correct option: 4 Explanation: Pneumonia is an infection that inflames the air sacs on one or both the lungs. It is the most common cause for consolidation in the lungs.
Q51) The ________ brings about dilation of blood vessels? 1. Histamine 2. Gastrin 3. Elastin 4. Heparin
Correct option: 1 Explanation: when histamine is released from its granules it causes dilation of blood vessels, which increases the permeability and the blood pressure is lowered.
Q50) Which one of the following lichens are used to obtain usnic acid? 1. Usnea and Cladonia 2. Evernia and Ramalina 3. Rocella and Lassalia 4. Usnea and Citraia
Correct option: 1 Explanation: Usnea and Cladonia are used to obtain usnic acid.
Q49) Which one of the following blood vessels carry oxygenated blood? 1. Coronary sinus 2. Coronary artery 3. Pulmonary artery 4. Coronary vein
Correct option: 2 Explanation: Coronary artery carries oxygenated blood to the heart muscle. Cardiac veins drain away the blood after it has been deoxygenated.
Q48) Wine and beer are produced without _________ . 1. Distillation 2. Malting 3. Fermentation 4. Mashing
Correct option: 1 Explanation: Wine and beer are produced without distillation. They are produced by fermentation, as distillation increases the alcohol content in the drink.
Q47) Which one of the following animal has longer loop of Henle? 1. Camel 2. Rat 3. Monkey 4. Labeo
Correct option: 1 Explanation: Camel has long loop of Henle. Desert animals do not find water easily, so they excrete less amount of water. Longer the henle’s loop, mor ethe solute will be...
Q46) Which one of the following is a heteropolysaccharide? 1. Cellulose 2. Starch 3. Glycogen 4. Hyaluronic acid
Correct option: 4 Explanation: Hyaluronic acid is a heteropolysaccharide. Heteropolysaccharides are polysaccharides whose molecules are composed of various types of monosaccharides
Q45) Gaurav visited a zoo where he saw tiger, lion. Camel, musk deer, red fox, one-horned rhinoceros, great Indian bustard and peacock. He wanted to report the endangered species from the above list/The numbers will be ___________ 1. 3 2. 4 3. 5 4. 8
Correct option: 2 Explanation: The correct number of endangered species is 4. One horned rhino, huge Indian Bustard, Red fox, and musk deer are all endangered species, Species that are in grave...
Q44) Which one of the following is cause of inflammation of cornea, called snow blindness cataract? 1. The gamma rays 2. High dose of cosmic rays 3. High dose of UV-B rays. 4. The infrared rays.
Correct option: 3 Explanation: High dose of UV-B rays causes inflammation of cornea, called snow blindness cataract. Snow blindness is a painful, temporary loss of vision because of overexposure to...
Q43) Proximal convoluted tubule differs from distal convoluted tubule being lined by ___. 1. Squamous cells with few microvilli 2. Squamous cells with many microvilli 3. Cuboidal cells with few microvilli 4. Cuboidal cells with many microvilli
Correct option: 4 Explanation: Proximal convoluted tubule differs from distal convoluted tubule by being lined by Cuboidal cells with many microvilli. PCT is lined by brush border cuboidal cells...
Q42) Breasts are the modified ________ glands. 1. Sebaceous 2. Ceruminous 3. Sweat 4. Vestibular
Correct option: 3 Explanation: Breasts are the modified sweat glands. Functionally mammary glands produce milk, but structurally they are modified sweat glands.
Q41) Select the correct statement. 1. The ovum secretes antifertilizin 2. The sperm secretes antifertilizin. 3. The zygote secretes fertilizin. 4. The sperm secretes fertilizin
Correct option: 2 Explanation: The sperm secretes antifertilizin. An antifertilizin is an acidic protein secreted by the plasma membrane of the sperm.
Q40) Empirical formula of chlorophyll -a is _________ . 1. C55H72O4N5Mg 2. C72H55O5N4Mg 3. C55H70O5N4Mg 4. C55H72O5N4Mg
Correct option: 4 Explanation: Empirical formula of chlorophyll -a is C55H72O5N4Mg. The chlorophyll molecule consists of a central magnesium atom surrounded by a nitrogen containing structure called...
Q39) Excessive haemolysis of RBCs with over production of bilirubin and abnormal function of _______ is observed in jaundice. 1. Lungs 2. Heart 3. Liver 4. Stomach
Correct option: 3 Explanation: Excessive haemolysis of RBCs with over production of bilirubin and abnormal function of liver is observed in jaundice.
Q38) Select the incorrect statement 1. female bird is heterogametic 2. holandric genes are present on n0n-homologous region of Y chromosome. 3. Queen bee and worker bees have haploid number of chromosomes. 4. Father is responsible for sex of the child in human beings.
Correct option: 3 Explanation: Queen bee and worker bees do not have haploid number of chromosomes. Queen bee and worker bees have diploid number of chromosomes.
Q37) Internal valves are present in the ________ . 1. Arterioles 2. lymphatic vessels 3. vascular capillaries 4. lymph capillaries
Correct option: 2 Explanation: Internal valves are present in the lymphatic vessels. These valves prevent backflow of fluid, so that lymph flows forward and not falls backward.
Q36) Taq polymerase isolated from Thermus aquaticus can withstand temperature up to ________ . 1. 84°C 2. 91°C 3. 94°C 4. 60°C
Correct option: 3 Explanation: Taq polymerase isolated from Thermus aquaticus can withstand temperature up to 94°C. It is so because this much temperature is needed for DNA strand separation without...
Q35) Hydra and yeast reproduce asexually by _________ . 1. Binary fission 2. Budding 3. Zoospores 4. Conidia
Correct option: 2 Explanation: Hydra and yeast reproduce asexually by budding. Formation of a bud takes place on the body of hydra and cells of yeast. It grows and when reaches a specific size it...
Q34) Match the process in column I with its explanation in column II and select the correct option. Column I column II i) Symport a) shrinking of protoplasm in a plant cell when placed in hypertonic solution. ii) Facilitated diffusion b) transport of two types of molecules in same direction across cell membrane iii) Antiport c) selective transport of molecules across the membrane through proteins iv) Plasmolysis d) selective transport of molecules in opposite direction across cell membrane 1. (i) – a, (ii) – d, (iii) – c, (iv) – b, 2. (i) – b, (ii) – c, (iii) – d, (iv) – a, 3. (i) – b, (ii) – a, (iii) – c, (iv) – d, 4. (i) – b, (ii) – a, (iii) – c, (iv) – d,
Correct option: 2 Explanation: Symport means transport of two types of molecules in the same direction across cell membrane. Facilitated diffusion is selective transport of molecules across the...
Q33) In ornithophilous plants, flowers lack ______ . 1. Sticky pollen grains 2. Nectar 3. Bright color 4. Fragrance
Correct option: 4 Explanation: In ornithophilous plants, flowers lack fragrance. Such flowers secrete profuse dilute nectar. They do not have any smell as birds have a poor sense of smell.
Q32) Mortality of a region is assesses by ______ individuals per unit area per unit time. 1. Births 2. Immigration 3. Emigration 4. Deaths
Correct option: 4 Explanation: Mortality of a region is assesses by deaths individuals per unit area per unit time. The number of fatalities in a given area per unit of time is referred to as...
Q31) In a population of an organism, allele A has the frequency of 0.7 and allele a has the frequency of 0.3 . What is the frequency of homozygous dominant allele? 1. 0.49 2. 0.14 3. 1.4. 4. 4.9
Correct option: 1 Explanation: The frequency of homozygous dominant allele will be 0.49
Q30) Which of the following is not involved in glycolysis? 1. Release of water 2. Utilization of ATP 3. Formation of ATP 4. Release of CO2
Correct option: 4 Explanation: Release of CO2 is not involved in glycolysis. Glycolysis takes place in cytoplasm, and produces 2ATP and 6NADH.
Q29) With reference to human beings, find out the mis-match pair. 1. Insemination – discharge of semen into the vagina of a female. 2. Implantation – setting of zygote in the endometrium uterus 3. Menopause – total arrest of menstrual cycle forever. 4. Menarche – beginning of menstrual cycle for the first time in life
Correct option: 1 Explanation: Implantation is the attachment of the fertilized egg to the uterine lining. The zygote usually implants at the top of the uterus, near where it exits the fallopian...
Q28) Gargi constructed m-RNA in laboratory. Which of the following codons will she have as initiating and terminating the m-RNA respectively. 1. AUG – UAG 2. GUG – UAC 3. UAC – AUG 4. AUG – UCA
Correct option: 1 Explanation: AUG – UAG will be the codons as initiating and terminating the m-RNA respectively.
Q27) Cretaceous, Jurassic and Triassic periods are included in the _______ era. 1. Palaeozoic 2. Mesozoic 3. Proterozoic. 4. Cenozoic
Correct option: 2 Explanation: Cretaceous, Jurassic and Triassic periods are included in the Mesozoic era. Mesozoic means middle life and this is the time of the dinosaurs. During this era, the land...
Q26) Match the correct phenotype and genotype of Drosophilia for their wing sizes. Phenotype Genotype i) Normal wings a) vgno ii) Nicked wings b) vg iii) Notched wings c) vg+ iv) Strap wings d) vgst v) Vestigial wing e) vgni 1. (i)-e, (ii)-d, (iii)-c, (iv)-b, (v)-a 2. (i)-a, (ii)-b, (iii)-c, (iv)-d, (v)-e 3. (i)-d, (ii)-a, (iii)-b, (iv)-e, (v)-c 4. (i)-c, (ii)-e,MHCET(iii)-a, (iv)-d, (v)-b
Correct option: 4 Explanation: In Drosophilia normal wings are related to vg+( vestigial wings ). In Drosophilia nicked wings are related to vgni In Drosophilia notched wings are related to vgno In...
Q25) Lemurs are found in ________ . 1. Central America 2. East Indies 3. Madagascar 4. South Africa
Correct option: 3 Explanation: Lemurs are primates present mainly on the African island of Madagascar. Lemurs include monkeys, apes, and humans.
Q24) With reference to agricultural crop, which of the following are considered as critical elements? 1. Mg, Fe, Zn 2. C, H, O 3. N, P, K 4. Mn, Cl, Ca
Correct option: 3 Explanation: N, P, K are considered as critical elements. NPK, are big 3 primary nutrients in commercial fertilizers. Nitrogen is considered to be the most important nutrient.
Q23) Water present in the form of hydrated oxides of silicon and aluminum in soil is called ______ water. 1. Gravitational 2. Capillary 3. Hygroscopic 4. Combined
Correct option: 4 Explanation: Water present in the form of hydrated oxides of silicon and aluminum in soil is called combined water. The water chemically combined in the structure of soil minerals...
Q22) A 22 year old girl is about to face interview. She is restless, is sweating and her heart beats have increased. These symptoms are due to increases secretion mainly of ________ . 1. Thymosins 2. Catecholamines 3. Aldosterone 4. Androgens
Correct option: 2 Explanation: The symptoms are due to increases secretion mainly of catecholamine. Catecholamines are group of similar substances released into the blood in response to physical or...
Q21) The dead leucocytes are destroyed in the following organs/ fluid except _______ . 1. Spleen 2. Lymph node 3. Blood 4. Liver
Correct option: 1 Explanation: The dead leucocytes are destroyed in the following organs/ fluid except spleen. Spleen is the part of the lymphatic system, which fight against infections and balances...
Q20) In angiospermic flowers, the filament of stamen is attached to the anther by _______ . 1. Placenta 2. Hilum 3. Funicle 4. Connective
Correct option: 4 Explanation: In angiospermic flowers, the filament of stamen is attached to the anther by connective. The sterile tissue between the lobes is called the connective, an extension of...
Q19) Who postulated three laws that known as Mendel’s law of inheritance based on Mendel’s findings? 1. Hugo De Vries 2. Karl Correns 3. Johannsen 4. Erich Tschermak
Correct option: 2 Explanation: Karl Correns postulated three laws, known as Mendel’s law of inheritance based on Mendel’s findings. Mendel’s law of inheritance consists of law of dominance, law of...
Q18) The subaerial branch which creeps horizontally on soil helps in vegetative propagation is called _______ . 1. Runner 2. Offset 3. Stolon 4. Sucker
Correct option: 4 Explanation: Sucker is the subaerial branch which creeps horizontally on soil helps in vegetative propagation. Sucker is present in plant as the modified stems.
Q17) Which one of the following is not characteristics of plasmid? 1. Help bacteria survive and reproductive under unfavorable conditions 2. Double stranded 3. Self- replicating 4. Heredity material of bacterium
Correct option: 4 Explanation: Heredity material of bacterium is not a characteristics of plasmid. Plasmid are extrachromosomal circular DNA molecules which are not part of the bacterial genome.
Q16) Match the column I with column II and select the correct option. Column I column II a) Female banded krait i) Humulin b) Pancreas from dog ii) DNA sample c) Hair root of human iii) insulin extracted and purified d) E.coli iv) DNA probe 1. a-i b-ii c-iv d-iii 2. a-ii b-I c-iii d-iv 3. a-iii b-iv c-ii d-i 4. a-iv b-iii c-ii d-i
Correct option: 4 Explanation: A segment of DNA was isolated from sex determining Y chromosome of female banded krait, an Indian poisonous snake. Insulin extracted from dog must be purified to...
Q15) The shortest phase of cardiac cycle is ______ . 1. Joint cardiac diastole 2. Ventricular systole 3. Atrial diastole 4. Atrial systole
Correct option: 4 Explanation: The shortest phase of cardiac cycle is atrial systole. The atrial systole is the last phase of a diastole during which the ventricular filling is completed.
Q14) Proteins attached with a prosthetic group are called ________ proteins. 1. Structural 2. Simple 3. Contractile 4. Conjugated
Correct option: 4 Explanation: Proteins attached with a prosthetic group are called conjugated protein. A conjugated protein is defined as a protein to which another chemical group is attached by...
Q13) What is true about C4 plants _______ . 1. C3 pathway reactions take place in bundle sheath chloroplast and C4 pathway reactions in mesophyll chloroplast. 2. Both reactions occur in mesophyll chloroplast. 3. Both reactions occur in bundle sheath chloroplast. 4. C3 pathway reactions take place in mesophyll chloroplast and C4 pathway reactions in bundle sheath chloroplast.
Correct option: 1 Explanation: In C4 plants, C3 pathway reactions take place in bundle sheath chloroplast and C4 pathway reactions in mesophyll chloroplast. In C4 plants, photosynthesis takes place...
Q12) Fructose is contributed to the semen by ____ . 1. Seminal vesicles 2. Testis 3. Prostate gland 4. Cowper’s gland
Correct option: 1 Explanation: Fructose is contributed to the semen by seminal vesicles. Fructose is the primary source of energy for the spermatozoa within the ejaculate. Seminal vesicles produces...
Q11) Specialized cells that are sensitive to vibration, pain and tension are called ______ . 1. Proprioceptors 2. Baroreceptors 3. Statoacoustic receptors 4. Frigidoreceptors
Correct option: 1 Explanation: Proprioceptors are specialized cells that are sensitive to vibration, pain, and tension. Proprioceptors are sensory receptors placed in the subcutaneous tissues. They...
Q10) What will be the genotype of a carrier individual who shows sickle cell anaemic trait? 1. HbB HbB 2. HbA HbS 3. HbS HbS 4. HbA HbA
Correct option: 4 Explanation: The genotype of a carrier individual who shows sickle cell anemic trait will be HbA HbA . A person with sickle cell trait inherits one normal allele and one...
Q9) The average life span of blood platelets is _____ days. 1. 3 – 4 2. 1 – 3 3. 11 – 15 4. 5 – 10
Correct option- 4 Explanation: The average life span of blood platelets is 5 – 10 days. Bone marrow residing megakaryocytes produce approximately 100 billion platelets per day.
Q8) The figure given below is T.S. of Azolla leaf, the filaments present in the cavity of the leaf are of _________ 1. Nostoc 2. Tolypothrix 3. Anabaena 4. Oscillatoria
Correct option- 3 Explanation: The filaments present in the cavity of the leaf are of anabaena. Anabaena are genus of nitrogen-fixing blue-green algae. They have beadlike or barrel shaped cells
Q7) Following are prokaryotic cells except _______. 1. Anabaena 2. Streptococcus 3. Nostoc 4. Paramecium
Correct option- 4 Explanation: Paramecium is not a prokaryotic cell, as it is a eukaryote. It has well-organized cells.
Q6) How many base pairs are present in a segment of m-RNA having 100 nucleotides? 1. 100 2. 50 3. 00 4. 25
Correct option- 3 Explanation: There are four nitrogenous bases in RNA: adenine, cytosine, uracil and guanine. 00 base pairs are present in a segment of m-RNA having 100 nucleotides
Q5) A molecule of chlorophyll which acts as reaction centre in pigment system II 1. P-650 2. P-700 3. P-673 4. P-680
Correct option- 4 Explanation: P-680 or photosystem II primary donor, is the reaction center chlorophyll a molecular diner associated with photosystem II in plants algae and cyanobacteria
Q3) The process of non-cyclic photophosphorylation occurs in ________. 1. Aerobic conditions, high CO2 conc. And low light intensity. 2. Anaerobic conditions, high CO2 conc. and enough light intensity. 3. Aerobic conditions, high CO2 conc. and enough light intensity. 4. Aerobic conditions, low CO2 conc. and enough light intensity.
Correct option- 3 Explanation: In the process of photosynthesis, the phosphorylation of ADP to form ATP by using the energy of sunlight is known as photophosphorylation.
Q2) Which one of the following molecule is not needed during translation in protein synthesis? 1. m-RNA 2. DNA 3. R-RNA 4. T-RNA
Correct option- 2 Explanation: DNA is not needed during translation in proteins. The work for DNA is to store information in the nucleus and function as the template
A microscope will have maximum resoving power, if to illuminate the specimen, it uses light of
1. red colour 2. green colour. 3. yellow colour. 4. blue colour. Solution: blue colour The resolving power of an instrument refers to its ability to resolve two points that are close together. We...
In balanced metre bridge 5Ω is connected in the left gap and RΩ in the right gap. When RΩ is shunted with an equal resistance, the new balance point is at 1.6 l1 where ‘l1 is the earlier balancing length. The value of ‘l1is
25 cm 40 cm 35 cm 30 cm Solution: 25 cm Using the meter bridge balance condition and putting the known values, we can write for the above circuit as:
Calculate the value of E, for given circuit, when value of 2 amp current is either flowing in clockwise or anticlockwise direction
3V, 28V 38V, 2V 3V, 30V 3V, 25V Solution: 38V, 2V Case 1: Clockwise Direction Applying Kirchoff's loop rule in the above circuit, we get: $ E-\left( 6\times 2 \right)-20-3\times 2=0 $ $ E=38\,V $...
A positively charged particle (q) travelling at 30° with respect to the direction of magnetic field of strength 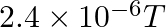 experiences a force of
experiences a force of 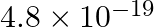 N. The speed of charged particle will be
N. The speed of charged particle will be
Solution: Option 2 is correct $ We\,know\,that: $ $ F=Bqvsin\theta $ $ \Rightarrow v=\frac{F}{Bqsin\theta } $ $ Putting\,the\,given\,values,\,we\,get: $ $ v=\frac{4.8\times {{10}^{-19}}}{2.4\times...
In resonance tube, the first and second resonance are heard when water level is 24.1 cm and 74.1 cm respectively, below the open end of the tube. The inner diameter of the tube is
1. 5 cm 2. 3 cm 3. 4 cm 4. 2 cm Solution: 3 cm We have, end correction in a resonance tube is, e = ( l2 − 3l1 )/2 given l1 = 24.1cm, l2 = 74.1cm and e = 0.3d , (d=diameter of tube )...
The fundamental frequency of a string stretched with a weight ‘M’ kg is ‘n’ , hertz. Keeping the vibrating length constant, the weight required to produce its octave is
1. M 2. 8 M 3. 2 M 4. 4 M Solution: 4M Octave means that the frequency is in the ratio : n':n = 2:1 We know that the frequency is directly proportional to the tension. It is given as follows: $...
A string of length ‘I’ and linear density ‘m’ has a fundamental frequency ‘n’ when stretched by tension ‘T’. The fundamental frequency of another string having double the length and double linear density, when same tension is applied is
$ 1.\,\,\frac{n}{2\sqrt{2}} $ $ 2.\,\,2n $ $ 3.\,\,n/2 $ $ 4.\,\,n/\sqrt{2} $ Solution: $ 1.\,\,\frac{n}{2\sqrt{2}} $
Solution: Option 3 is correct $ We\,have:\,\,\overrightarrow{A}={{a}_{1}}\widehat{i}+{{a}_{2}}\widehat{j}+{{a}_{3}}\widehat{k},\, $ $ \,\widehat{i}\times \left( \widehat{i}\times \overrightarrow{A}...
The Young’s double-slit experiment is performed with the light of blue colour (4350 A) and then with green colour (5450 A). Without changing experimental setup, if the distance of the sixth fringe from the centre is determined for both the colours as Xblue and Xgreen • then Xbiue : X green is nearly
0.2 1.2 0.8 1.5 Solution: 0.8 $ We\text{ }know\text{ }that~ $ $ Distance\text{ }of\text{ }the~nth~bright\text{ }fringe~is\,given\,by: $ $ {{y}_{n}}=\frac{n\lambda D}{d} $ $ \Rightarrow...
Assuming the earth to be a sphere of uniform density, the ratio of acceleration due to gravity on the earth’s surface to its value at halfway towards the centre of the earth, will be
2 : 1 2 : 3 1 : 1 1 : 2 Solution: 2 : 1 Let g and g’ be the acceleration due to gravity on the earth's surface and at a depth d from the surface respectively. The, we can write: $ g'=g\left(...
A stone of mass 2kg attached at one end of the of a 2m long string is whirled in horizontal circle. The string makes an angle of 45 degrees with the vertical then the centripetal force acting on the string is
30 N 40 N 20 N 10 N Solution: 20N $ Given: $ $ m=2kg,\,r=2m,\,\,\theta ={{45}^{\circ }} $ $ Now,~\,Fsin\theta =\frac{m{{v}^{2}}}{r} $ $ and\,v=\sqrt{rg\tan \theta } $ $ v=\sqrt{2\times 9.8\times...
4.5 5.5 6.5 2.5 Solution: 6.5 $ \left( 2\overrightarrow{{{A}_{1}}}+\overrightarrow{{{A}_{2}}} \right).\left( \overrightarrow{{{A}_{1}}}-\overrightarrow{{{A}_{2}}} \right) $ $...
For a transistor 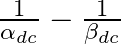 is (
is (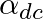 and
and 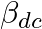 are current gains)
are current gains)
0 -1 1 2 Solution: 1 We have: $ \frac{1}{{{\alpha }_{dc}}}-\frac{1}{{{\beta }_{dc}}} $ $ We\,can\,write: $ $ \frac{1}{{{\alpha }_{dc}}}-\frac{1}{{{\beta }_{dc}}}=\frac{{{\beta }_{dc}}-{{\alpha...
Q1) Match the column I with column II and select the correct option. Column I column II A. Fibrinogen i) contractions in female reproductive tract B. Fructose ii) prevent fungal infection C. Prostaglandins iii) coagulation of semen D. Lactobacilli in vagina iv) source of energy 1. A(ii), B-(iii), C(iv), D(i) 2. A-(i), B(ii), C(iii), D(iv) 3. A(iii), B(iv), C(i), D(ii) 4. A(iv), B(i), C(ii), D(iii)
Solution: correct option – 3 Explanation: Fibrinogen helps in the coagulation of semen after ejaculation. Fructose is a source of energy. Prostaglandins help in contractions in the female...
How many moles of ethyl bromide are required to produce ‘n’ moles of butane by Wurtz synthesis ?
1. 3n 2. n 3. 2n 4. n/2 Solution: 2n When two moles of ethyl bromide are combined with Sodium metal in the presence of dry ether, n-butane is formed. The reaction described above is known as the...
The number of sigma and Pi bonds respectively present in a molecule of toluene is,
10σ and 3π bonds 12σ and 3π bonds 15σ and 3π bonds 6σ and 3π bonds Solution: 15σ and 3π bonds Toluene is C6H5CH3. No. of σ bonds is 15. No. of π bonds...
Which of the following is not basic alpha-amino acid?
1. Histidine 2. Lysine 3. Arginine 4. Alanine Solution: Alanine Except Alanine all other amino acids contain - NH2 or - N atom in the side chain.
Which of the following methods of refining of crude metals uses green logs of wood ?
1. Zone refining 2. Polling 3. Liquation processes 4. Vapour phase refining Solution: Polling Poling is a crude metal refining technique that involves stirring molten metal in a ladle with a green...
Enthalpy of fusion of water is 6.0 KJ/mol at 0 degree celsius. What is the enthalpy change when 0.0045 kg of water undergoes fusion?
1.5×10-2 kJ 1.5 kJ 150 kJ 1.5×10-3 kJ Solution: 1.5 kJ
One atom of an element ‘X’ weighs 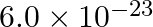 g. mass of 1 mole of atoms is
g. mass of 1 mole of atoms is
40 mol-1 32.31 mol-1 4.5 mol-1 36.13 mol-1 Solution: 36.13 mol-1 The mass of one atom of an element, A = 6.0 × 10−23 g We know, 1 mole of an atom is 6.023 × 1023 atoms. 1 mole of an atom = its...
O- acetyl salicylic acid is used as
1. Antimicrobial 2. tranquilizer 3. antioxidant 4. antirhuematic drug Solution: antirhuematic drug Aspirin, commonly known as acetylsalicylic acid (ASA), is a pain reliever, fever reducer, and...
Haloform test is NOT exhibited by
1. Ethanal 2. Propanal 3. Propanone 4. Acetophenone Solution: Propanal Haloform reaction is given by the compounds containing acetyl groups (CH3CO−). All the given options...
Oraganic compounds ‘A’ and ‘B’ react with sodium metal and liberate hydrogen gas. ‘A’ and ‘B’ react together to give ethyl methanoate, compound A and B respectively are
Solution: Option 1 is correct
Predict the product of following reaction
A. 2-hydroxy cyclohexanol B. Cyclohexene C. Benzene D. 2-Bromo cyclohexene Solution: Cyclohexene An alkene (cyclohexene) will be created in this case by dehydrating alcohol...
A solution contains 1.2 moles of solute and 13.8 moles of solvent. Hence the mole fraction of the solute is
1. 0.085 2. 0.09 3. 0.08 4. 0.075 Solution: 0.08 The mole fraction is given as: $ {{X}_{solute}}=\frac{{{n}_{solute}}}{{{n}_{solute}}+{{n}_{solvent}}} $ $...
Acetic acid on treatment with NaOH gives Y and with SOCl2 gives Z. Y and Z when heated form acetic anhydride. The compounds Y and Z respectively are:
Solution: Option 3 is correct Sodium hydroxide (NaOH) reacts with acetic acid (CH3COOH) to form sodium acetate (CH3COONa) and water (H2O). The equation for this reaction can...
Calculate the volume of bcc type unit cell having edge length 288 pm and 7.2 g cm-3 density? (mass of unit cell= 52g)
$ 1.\,\,7.22\,c{{m}^{3}} $ $ 2.\,\,1.38\times {{10}^{-1}}\,c{{m}^{3}} $ $ 3.\,\,2.38\times {{10}^{-2}}\,c{{m}^{3}} $ $ 4.\,\,3.023\times {{10}^{-2}}\,c{{m}^{3}} $ Solution: $ 1.\,\,7.22\,c{{m}^{3}}...
What type of glycosidic linkage is present in maltose ?
1. 1 → 6 β 2. 1 → 4 β 3. 1 → 6 α 4. 1 → 4 α Solution: 1 → 4 α Maltose is a disaccharide made up of alpha 1,4 glucose molecules linked together. Maltase is the enzyme that breaks it down. It acts on...
Which of the following pairs of solutions will be isotonic at the same temperature?
1M NaCl and 1M MgCl2 1.5M KCl and 2.5M Urea 1.5M AlCl3 and 2M Na2SO4 1M NaCl and 2M MgCl2 Solution: 1.5M AlCl3 and 2M Na2SO4 The osmotic pressure and molar concentration in isotonic solutions should...
Identify optically inactive compound among the following
1. 2 - chloropropanal 2. 2 - chloropentane 3. 2 - chloro,2-methylbutane 4. 2 - chlorobutane Solution: 2 - chloro,2-methylbutane 2-chloro-2-methylbutane is optically inactive as no chiral center is...
Which among the following complex ion is NOT diamagnetic?
$ 1.\,\,{{\left[ Ni{{(CN)}_{4}} \right]}^{2-}} $ $ 2.\,\,[Ni{{(CO)}_{4}}] $ $ 3.\,\,{{\left[ CO{{(N{{H}_{3}})}_{6}} \right]}^{3+}} $ $ 4.\,\,{{[NiC{{l}_{4}}]}^{2-}} $ Solution: $...
The standard enthalaphy of formation of ethane is -84.7 KJ/mol. What is the enthalapy change for the formation of 0.06 kg ethane?
1. -42.35 kJ 2. -169.4 kJ 3. -50.82 kJ 4. -236.7 kJ Solution: -169.4 kJ Given mass = 0.06 kg = 60 g Molar mass of ethane = 2(12) + 6(1) = 30 g number of moles of ethane in 60 grams = 60/30 = 2 We...
What happens during the action of catalyst?
1. Temperature increases 2. Temperature decreases 3. Ea increases 4. Ea decreases Solution: Ea decreases A catalyst is a material that speeds up a reaction without being consumed in the process....
Styrene and 1,3-butadiene on polymerisation forms
1. Buna-N 2. Neoprene 3. Butyl rubber 4. Buna-S Solution: Buna-S Styrene-butadiene rubber is another name for the rubber produced (SBR). Bu stands for butadiene, Na for sodium, and S for styrene in...
Construct a triangle with sides 3 cm, 4 cm and 5 cm. Draw its circumcircle and measure its radius.
Solution: Steps to construct: Step 1: Draw a line segment BC = 4cm. Step 2: With Center as B and radius 3cm, with center as C and radius 5cm draw two arcs which intersect each other at point A. Step...
Using a ruler and a pair of compasses only, construct: (i) A triangle ABC given AB = 4 cm, BC = 6 cm and ∠ABC = 90°. (ii) A circle which passes through the points A, B and C and mark its centre as O. (2008)
Solution: Steps to construct: Step 1: Draw a line segment AB = 4cm. Step 2: At point B, draw a ray BX making an angle of 90o and cut off BC = 6cm. Step 3: Join AC. Step 4: Draw the perpendicular...
Draw an equilateral triangle of side 4 cm. Draw its circumcircle.
Solution: Steps to construct: Step 1: Draw a line segment BC = 4cm. Step 2: With centers B and C, draw two arcs of radius 4cm which intersects each other at point A. Step 3: Join AB and AC. Step 4:...
Draw a line segment AB of length 8 cm. Taking A as centre, draw a circle of radius 4 cm and taking B as centre, draw another circle of radius 3 cm. Construct tangents to each circle from the centre of the other circle.
Solution: Steps to construct: Step 1: Draw a line segment AB = 8cm. Step 2: With center as A and radius 4cm, with center as B and radius 3cm, draw circles. Step 3: Draw the third circle AB as...
The temperature and pressure of 4 dm3 of an ideal gas are doubled. The volume of the gas now is
2 dm3 3 dm3 4 dm3 8 dm3 Solution: 4 dm3 From ideal gas equation, we have: $ \frac{{{P}_{1}}{{V}_{1}}}{{{T}_{1}}}=\frac{{{P}_{2}}{{V}_{2}}}{{{T}_{2}}} $ $ We\,have\,: $ $...
PCl5 exists but NCl5 doesn’t, because of
1. NCl5 is unstable 2. larger size of nitrogen 3. inertness of nitrogen 4. non-availability of vacant d- atomic orbitals Solution: non-availability of vacant d- atomic orbitals By employing the...
Which among following raections of water generates oxygen gas ?
1. Reaction with calcium oxide 2. Reaction with sodium 3. Reaction with ammonia 4. Photosynthesis reaction Solution: Photosynthesis reaction The overall balanced equation of photosynthesis reaction...
Nitronic acid with 50% sulphuric acid at room temperature forms an aldehyde. This reaction is known as
1. Gabriel phthalamide synthesis 2. Nef carbonyl synthesis 3. Etard reaction 4. Hoffmann degradation Solution: Nef carbonyl synthesis Nef carbonyl synthesis Acid hydrolysis of a salt of a...
The sodium fusion extract of aniline is boiled with ferrous sulphate and then acidified with concentrated sulphuric acid. The color of the complex formed is
1. Violet 2. Yellow 3. Black 4. Prussian blue Solution: Prussian blue The reaction equation is: $ FeS{{O}_{4}}+NaOH\,\,\to \,\,Fe{{(OH)}_{2}}+N{{a}_{2}}S{{O}_{4}} $ $ 6NaCN+Fe{{(OH)}_{2}}\,\,\to...
Which among the following elements exhibits only +3 oxidation state?
Nd La Dy Yb Solution: La We have Z = 57 for La. In +3 oxidation state Lanthanum(La) achieves the noble gas configuration. So, Lanthanum exhibits only +3 oxidation state.
Any galvanic cell working under standard condition, if the equation of the cell reaction is multiplied by 3 then
A. Eo increase three times B. Eo is unchanged C. Eo decreases three times D. Go remains unchanged Solution: Eo is unchanged $ Nernst's\text{ }equation\text{ }states~: $ $...
(a) In the figure given below, O is the center of the circle. If ∠BAD = 30°, find the values of p, q and r.
(a) In the figure given below, two circles intersect at points P and Q. If ∠A = 80° and ∠D = 84°, calculate (i) ∠QBC (ii) ∠BCP Solution: (i) ABCD is a cyclic quadrilateral ∠A + ∠C = 180o 30o + p =...
What type of hybridization is present in PCl5 molecule?
A. sp3d2 B. sp3d3 C. sp3d D. dsp2 Solution: sp3d2 PCl5 is expected to have a hybridization of sp3d and a shape of trigonal bipyramidal. But in solid-state PCl5 exists in ionic form...
(a) In the figure given below, ABCD is a cyclic quadrilateral. If ∠ADC = 80° and ∠ACD = 52°, find the values of ∠ABC and ∠CBD.
(b) In the figure given below, O is the center of the circle. ∠AOE =150°, ∠DAO = 51°. Calculate the sizes of ∠BEC and ∠EBC. Solution: (a) In the given figure, ABCD is a cyclic quadrilateral ∠ADC =...
(a) In the figure, (i) given below, if ∠DBC = 58° and BD is a diameter of the circle, calculate: (i) ∠BDC (ii) ∠BEC (iii) ∠BAC
(b) In the figure (if) given below, AB is parallel to DC, ∠BCE = 80° and ∠BAC = 25°. Find: (i) ∠CAD (ii) ∠CBD (iii) ∠ADC (2008) Solution: (a) ∠DBC = 58° BD is diameter ∠DCB = 90° (Angle in...
Identify the amphoteric oxide from the following
B2O3 CO ZnO CaO Solution: ZnO Zinc oxide (ZnO) is known as amphoteric oxide because it has both acidic and basic properties.
(a) In the figure (i) given below, O is the center of the circle. If ∠AOC = 150°, find (i) ∠ABC (ii) ∠ADC (b) In the figure (i) given below, AC is a diameter of the given circle and ∠BCD = 75°. Calculate the size of (i) ∠ABC (ii) ∠EAF.
Solution: (a) Given, ∠AOC = 150° and AD = CD We know that an angle subtends by an arc of a circle at the center is twice the angle subtended by the same arc at any point on the remaining part of the...
If O is the center of the circle, find the value of x in each of the following figures (using the given information)
Solution: From the figure (i) ABCD is a cyclic quadrilateral Ext. ∠DCE = ∠BAD ∠BAD = xo Now arc BD subtends ∠BOD at the center And ∠BAD at the remaining part of the circle. ∠BOD = 2 ∠BAD = 2 x 2 x =...
If O is the center of the circle, find the value of x in each of the following figures (using the given information):
Solution: (i) ∠ACB = ∠ADB (Angles in the same segment of a circle) But ∠ADB = x° ∠ABC = xo Now in ∆ABC ∠CAB + ∠ABC + ∠ACB = 180o 40o + 900 + xo = 180o (AC is the diameter) 130o + xo = 180o xo =...
Which of the following allotropic forms of sulphur exists in chair form?
1. Cyclo - sulphur 2. α - Sulphur 3. β - Sulphur 4. plastic sulphur Solution: Cyclo - sulphur
The standard electode potential of calomel electrode is increased by
decreasing the concentration of KCl solution Increasing the quality of Hg2Cl2 decreasing the quantity of calomel Increasing the concentration of KCl solution Solution: decreasing the concentration...
What is the charge on 0.05 mol of electrons?
1. 2412.5 C 2. 965 C 3. 4825 C 4. 9650 C Solution: 4825 C We have: Mass of one electron = 9.10×10−31 kg Charge of one electron = 1.602×10−19 coulomb $...
Which is most stable oxidation state of Vanadium (Atomic no. 23)?
1. +2 2. +3 3. +4 4. +5 Solution: +5 Ammonium metavanadate, NH4VO3, is the most common source of vanadium in the +5 oxidation state. This isn't particularly water soluble, thus it's normally...
When aniline forms 2,4,6- tribromo aniline on reaction with bromine water , it undergoes
1. nucleophilic addition 2. nucleophilic substitution 3. electrophilic substitution 4. electrophilic addition Solution: electrophilic substitution Bromination is the reaction that is taking place...
For the elementary reaction 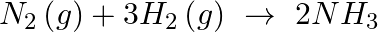 . Identify the correct relation from the following relations:
. Identify the correct relation from the following relations:
Solution: the correct option is A As the reaction progresses, the reactant concentration decreases and the product concentration rises. As a result, the rate of...
The decreasing order of thermal stability of hydrides of group 15 elements is
Solution: The correct option is 2. Hydride stability reduces as one moves along the group from NH3 to SbH3. This is because their bond dissociation enthalpy has decreased. The...
The magnitude of 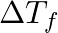 is
is
1. Nature of solute only 2. Nature of the solute and the concentration of solution 3. Nature of the solvent and the concentration of solution 4. Nature of solvent only Solution: 3. Nature of the...
What is SI unit of Luminous intensity?
1. Ampere 2. 0C 3. Kelvin 4. Candela Solution: Candela The SI unit of luminous intensity is candela.
Two moles of an ideal gas are expanded from volume of 15.5 litre to 20 litre against a constant external pressure of 1 atmosphere. The amount of work done is
- 4.5 J 2×10-2 J - 506.5 J 225 J Solution: - 506.5 J The following equation can be used to describe the work done by a system against an external pressure: We know that: w = -Pext ∆V 1atm...
Which of following is a mineral of aluminium?
1. Dolomite 2. Siderite 3. China clay 4. Calamine Solution: China Clay $ Dolomite-[CaMg{{(C{{O}_{3}})}_{2}}] $ $ Siderite-[FeC{{O}_{3}}] $ $ China\text{ }Clay-[Al2{{O}_{3}}.2Si{{O}_{2}}.2{{H}_{2}}O]...
Neo-pentyl alcohol is a –
1. Tertiary alcohol 2. Secondary alcohol 3. Primary alcohol 4. Dihydric alcohol Solution: Primary alcohol The primary alcohols are those that have only one alkyl group linked to the carbon atom of...
Which among following element has highest chemical reactivity?
1. Be 2. Mg 3. Sr 4. Ba Solution: Ba Because of its high reactivity, barium (Ba) is only found in nature in conjunction with other elements. Sulfate and carbonate are the most common compounds...
Which of the following rate expression is true for alkaline hydrolysis of methyl bromide ?
Solution: Option 1. is the correct answer Consider methyl bromide's alkaline hydrolysis to yield methanol. $ C{{H}_{3}}-Br+NaOH\,\,\xrightarrow{\Delta }\,\,C{{H}_{3}}-OH+NaBr $...
Linkage Isomers Ionization Isomers Geometrical isomers Coordination isomers Solution: Ionization isomers When the same molecular formula produces distinct ions in a solution, this is known as...
Which among following gases is readily adsorbed by activated charcoal ?
H2 SO2 N2 O2 Solution: SO2 SO2 is an easily liquefiable gas and easily liquefiable gases are adsorbed to a greater extent than elemental gases like N2, O2, and H2.
Which one of the following is a neutral oxide?
$ 1.\,A{{l}_{2}}{{O}_{3}} $ $ 2.\,{{N}_{2}}O $ $ 3.\,N{{a}_{2}}O $ $ 4.\,S{{O}_{2}} $ Solution: $ 2.\,{{N}_{2}}O $ Oxides that are neither acidic nor basic are known as neutral oxides. In other...
A polymer obtained from the monomers ethylene glycol and dimethyl terephthalate is
1. Nylon-6 2. Terylene 3. Bakelite 4. Nylon-6,10 Solution: terylene Ethylene glycol (1,2 ethanediol) and terephthalic acid are the monomers of terylene (1,4 benzene dicarboxylic acid). Terylene...
Identify diamagnetic ion from following. (Atomic no.of Na=11, Cu=29, Fe=26, Cr=24)
Cu2+ Fe3+ Na+ Cr3+ Solution: Na+ Cu2+ = 1s22s22p63s23p63d9 Fe3+ = 1s22s22p63s23p63d5 Cr3+ = 1s22s22p63s23p63d3 Na+ = 1s22s22p6 All the given ions except Na+ have unpaired electrons in their...
Identify the oxidising agent in following redox reaction
$ C{{l}_{2}}+2B{{r}^{-}}\,\to \,2C{{l}^{-}}+B{{r}_{2}} $ $ 1.\,C{{l}^{-}} $ $ 2.\,B{{r}^{-}} $ $ 3.\,B{{r}_{2}} $ $ 4.\,C{{l}_{2}} $ Solution: $ 4.\,C{{l}_{2}} $ Br is the reducing agent because it...
Among the following, an artificial sweetening agent which does not contain – CO – NH – bonding in molecule is
1. Sucralose 2. Alitame 3. Aspartame 4. Saccharine Solution: Sucralose Alitame, Aspartame and Saccharine all three contain the CO-NH linkage whereas Sucrolose contains the C-O linkage....
Which of the following nitro-alkane doesn’t react with nitrous acid?
2-methyl-2-nitropropane 2-nitropropane Nitroethane 1-nitropropane Solution: 2-methyl-2-nitropropane The structure of 2-methyl-2-nitropropane is as follows: We can see that...
Pumice stone is an example of
Solid Sol Emulsion Aerosol Solid foam Solution: Solid Foam Pumice stone is an example of solid foam. In this type of colloid, the dispersion medium is solid and the dispersion phase is...
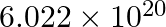 molecules of urea are present in 100 mL of its solution. The concentration of solution is
molecules of urea are present in 100 mL of its solution. The concentration of solution is
0.1 M 0.02 M 0.01 M 0.001 M Solution: 0.01 M Given: 6.02×1020 molecules of urea The volume of solution is 100/1000 = 0.1 L 1 mole of urea have 6.02×1023 molecules Number of moles present = 6.02×1020...
What products are expected from disproportionation reaction of orthophosphorus acid?
$ 1.\,\,{{H}_{3}}P{{O}_{3}}+P{{H}_{3}} $ $ 2.\,\,{{H}_{3}}P{{O}_{4}}+P{{H}_{3}} $ $ 3.\,\,P{{H}_{3}}+{{P}_{2}}{{O}_{5}} $ $ 4.\,\,{{H}_{3}}P{{O}_{3}}+{{P}_{2}}{{O}_{5}} $ Solution: $...
Identify the product B in following conversion!
$Chlorobenzene+{{H}_{2}}O\xrightarrow[\Pr essure]{Cu,\,673K}A\xrightarrow[373K]{conc.\,{{H}_{2}}S{{O}_{4}}}B$ 4-hydroxybenzene Sulphonic acid Benzene Sulphonic acid 2-hydroxybenzene Sulphonic acid...
What is the quantity of Gold chloride obtained when 4.5 g of gold and 2.1 g of Chlorine are sealed in a a tube and heated at 150 degreees C?
4.5 g 4.8 g 6.07 g 20.7 g Solution: 6.07 g Excess reagents are reactants that are not used up when a chemical reaction is completed. Because its quantity limits the amount of product generated, the...
What is the standard emf of following cell?
$ N{{i}_{(s)}}|(1M)\,Ni_{(aq)}^{2+}||(1M)\,Au_{(aq)}^{3+}|Au\left( s \right) $ $ if\,E_{Ni}^{\circ }=-0.25V,\,E_{Au}^{\circ }=1.50V $ -1.25 V 1.75 V 1.25 V -1.75 V Solution: 1.75 V $...
How many tertiary carbon atoms and primary carbon atoms respectively are present in 2-iodo-3, 3-dimethyl pentane?
2,4 0,4 2,3 1,3 Solution: 0,4 We can represent 2-iodo-3, 3-dimethyl pentane as follows: ${{H}_{3}}C-CH(I)-C{{(C{{H}_{3}})}_{2}}-C{{H}_{2}}-C{{H}_{3}}$ As we can see that there are zero carbon atoms...
5600 sec 360.0 sec 560.0 sec 3364 sec Solution: 560 sec $ Given: $ $ {{[R]}_{0}}=0.0210\,M~ $ $ [R]=0.0150\,M~ $ $ k=6\times {{10}^{-4}}\,{{\sec }^{-1}} $ $ For\text{ }a\text{ }first\text{...
Q100. Heaviness with severe chest pain which may disappear with rest indicates A) Angina pectoris B) Atherosclerosis C) Arteriosclerosis D) Hyperthyroidism
Correct option: A Explanation: Heaviness with severe chest pain which may disappear with rest indicates Angina Pectoris. It is a type of chest pain resulting because of decrease in blood flow to...
Q99. The increase in blood flow to heart stimulates secretion of A) Renin B) Oxytocin C) Antidiuretic hormone D) Atrial natriuretic factor
Correct option: D Explanation: The increase in blood flow to heart stimulates secretion of Atrial natriuretic factor. It is a protein hormones secreted by cardiac myocyte cells. Its function is the...
Q98. Which of the following animal was selected by Morgan for studying linkage ? A) Apis indica B) Agrobacterium tumafaciens C) Drosophila melanogaster D) E. Coli
Correct option: C Explanation: Drosophila melanogaster animal was selected by Morgan for studying linkage. He showed how sexual reproduction gave rise to variations.
Q97. The marine fish among the following varieties is A) Stromateus B) Labeo C) Cirrhina D) Catla
Correct option: A Explanation: Salt water fish are called as marine fish are those which live in ocean. Stromateus is a marine fish.
Which of the following alcohols is not having Csp3-OH bond?
Phenyl Methanol 2-Methyl Propan-2-ol Propan-2-ol Vinyl Alcohol Solution: Vinyl Alcohol Vinyl Alcohol is represented as follows: The simplest enol is vinyl alcohol, commonly...
Q96. Juxta glomerular cells of kidney secrete hormone A) Angiotensinogen B) Angiotensin II C) Coherin D) Renin
Correct option: D Explanation: Renin is an enzyme secreted by the juxta glomerular cells of the kidney. Renin is secreted in response to stimulation of beta-1-adrenergic response.
Q95. Epicanthal skin fold and simian crease are characteristics of A) Down’s syndrome B) Klinefelter’s syndrome C) Thalassemia D) Turner’s syndrome
Correct option: A Explanation: Down syndrome is an autosomal condition which is characterized by outward slanting eyes that have an additional skin fold on the upper eyelid recognized as an...
What is the number of hydroxyl group present in lactic acid?
Zero Three Two One Solution: One Lactic acid (2-hydroxy propionic acid) is a bifunctional molecule that has both a carboxylic acid and a hydroxyl group, making it useful in a...
Which of the following is not an octahedral complex?
$ 1.\,\,{{[Ir{{({{C}_{2}}{{O}_{4}})}_{2}}C{{l}_{2}}]}^{3-}} $ $ 2.\,\,{{[CoC{{l}_{2}}{{(en)}_{2}}]}^{+}} $ $ 3.\,\,{{\left[ Co{{(en)}_{2}}{{(N{{O}_{3}})}_{2}} \right]}^{+}} $ $ 4.\,\,\left[...
Q94. Helper T – cells : Lymphokines as Killer T – cells : _____ A) Interferons B) Lysozymes C) Perforins D) Prostaglandins
Correct option: C Explanation: As lymphokines are protein mediators produced by T cells to direct the immune system response by signaling between its cell. Killer T cells carry out their killing...
What is the oxidation state of iron in potassium ferrate?
+3 +4 +6 +2 Solution: +6 Potassium ferrate has the chemical formula K2FeO4. This purple salt is paramagnetic, and is a rare example of an iron(VI) compound. $...
Q93. Ectoderm gives rise to A) cornea, heart, bronchi, dentine B) adrenal cortex, tongue, liver, retina C) lungs, adrenal medulla, dermis, thyroid D) enamel of teeth, nails, adrenal medulla, hair
Correct option: D Explanation: Ectoderm gives rise to enamel of teeth, nails, adrenal medulla. Ectoderm is a germ layer, formed in an animal embryo during developmental stages. It is one of the...
Q92. The Human Genome Project (HGP) was initiated in A) 1988 B) 1990 C) 1992 D) 1994
Correct option: B Explanation: the human genome program was initiated in 1990. The program refers to thirteen year effort, which began in 13 October 1990 and completed in2003.
Q91. A Red list of endangered species is maintained by A) CSIR B) IUCN C) NEERI D) WLS
Correct option: B Explanation: IUCN (international union for conservation of nature) maintains a list of endangered species. It uses a set of quantitative criteria to evaluate the extinction risk of...
What is the number of moles of Silver Chloride precipitated when excess of aqueous silver nitrate is treated with ![Rendered by QuickLaTeX.com [Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7f8a1797a451d4bad014fffc46745e19_l3.png)
1 mole 2 mole 3 mole 4 mole Solution: 1 mole Only one mole of AgCI is precipitated with an excess of AgNO3 because only one Cl- is in the ionization sphere.
Q90. Choose the CORRECT group of labellings A) I – Trophoblast, II – Archenteron, III – Micromeres B) I – Trophoblast, II – Blastocoel, III – Megameres C) I – Trophoblast, II – Archenteron, III – Inner mass cells D) I – Trophoblast, II – Blastocoel, III – Inner mass
Correct option: D Explanation: The trophoblast is the outer layer of the blastocyst consisting of cells. It surrounds the inner cell mass and a fluid-filled cavity known as blastocoel.
Q89) Match the following : i. Mercury a. Low blood pressure, blindness ii. Lead b. Hyperkeratosis, Liver cirrhosis iii. Arsenic c. Bone deformation, testicular atrophy iv. Cadmium d. Abdominal pain, haemolysis e. Anaemia, convulsions A) i-e, ii-d, iii-c, iv-b B) i-d, ii-e, iii-b, iv-c C) i-c, ii-b, iii-d, iv-a D) i-b, ii-c, iii-d, iv-e
Correct option: B Explanation: Mercury if ingested causes abdominal pain. Lead causes anaemia and convulsions. Excessive intake of arsenic leads to liver cirrhosis. Cadmium causes abdominal leads to...
Q88) The Transgenic animals are generally produced for all of the following needs except A) Testing of chemical safety B) Testing of vaccine safety C) Stimulation of pathogenicity D) Production of pharmacologically important Proteins
Correct option: C Explanation: The Transgenic animals are generally produced for all of the following needs except for stimulation of pathogenicity. The transgenic animals are used for numerous...
Q87) Select the CORRECT identification group of labelled parts I, II and III A) I – Scala vestibuli, II – Scala media, III – Scala tympani B) I – Scala vestibuli, II – Scala tympani, III – Scala media C) I – Scala tympani, II – Scala media, III – Scala vestibuli D) I – Scala media, II – Scala tympani, III – Scala media
Correct option:A Explanation: Scala vestibuli, Scala media, and Scala tympani are located in the cochlea.
Q86) The reptiles, like dinosaurs were dominant in _____ period. A) Cretaceous B) Jurassic C) Tertiary D) Triassic
Correct option: B Explanation: The reptiles, like dinosaurs were dominant in Jurassic period. Jurassic is a geologic period
Q85) Find the CORRECT match : Column A Column B Column C i. Mackeral Rastrelliger Freshwater fish ii. Honey bee Apis Wax iii. Mirgala Tacchardia Marine waterfish iv. Silkworm Bombyx Mulberry silk A) ii and iv B) i and ii C) iv only D) i and III
Correct option: A Explanation: The honey bee is an insect within the genus Apis. Their produce was also known as beeswax. Silkworm belongs to the genus Bombyx and produces mulberry silk.
Q84) In the given diagram I and II indicate A) Chromomere and chromonemata B) Centromere and secondary constriction C) Secondary constriction and satellite D) Telomere and satellite
Correct option: D Explanation: I and II indicate Telomere and satellite. Telomere is the end of chromosome and are made of repetitive sequencing of non-coding DNA.
Q83) The glycoprotein, fertilizin is secreted by Ovum B) Ovary C) Sperm D) Testis
Correct option: A Explanation: The glycoprotein, fertilizin is secreted by ovum. Fertilizin is secreted by ovum, which forms egg water and attracts the sperms of its own species.
Q82) Deposition of _______ in the joints causes gout. A) Urea B) Uric acid C) Guanine D) Ammonia
Correct option: B Explanation: Deposition of uric acid in the joints causes gout. Gout is a disorder characterized by deposition of the uric acid crystals. This increases the pain and inflammation...
Q81) The characters such as pointed elongated snout, strong and stout forelimbs, well developed claws are observed in ………… adaptation. A) Arboreal B) Aerial C) Cursorial D) Fossorial
Correct option: D Explanation: The characters such as pointed elongated snout, strong and stout forelimbs, well developed claws are observed in fossorial adaptation. Fossorial adaptation refers to...
Q80) A cuckoo laying eggs in the nest of other species of birds, is an example of A) Adelphoparasitism B) Broodparasitism C) Ectoparasitism D) Hyperparasitism
Correct option: B Explanation: A cuckoo laying eggs in the nest of other species of birds, is an example of Brood parasitism. Brood parasites are organisms who rely on others to raise their young...
Q79) Which is NOT the function of lymph ? A) Transport R.B.C.s B) Drain excess tissue fluid C) Transport lymphocyte and antibodies D) Transport absorbed fat
Correct option: A Explanation: Transportation of R.B.C.s is not the function of lymph. R.B.C.s are a type of blood cells whose role is to transport oxygen from lungs to the body.
Q78) Pick the ODD homologous pair out. A) Bartholin’s Gland – Cowper’s Gland B) Clitoris – Penis C) Mons pubis – Glans penis D) Labia majora – Scrotum
Correct option: C Explanation: Mons pubis – Glans penis is the odd homologous pair. Mons pubis structure corresponds to shaft in male and not to glans penis.
Q77) More than 95 % of transgenic animals are A) Rabbits B) Mice C) Fish D) Cows
Correct option: B Explanation: More than 95 % of transgenic animals are mice. Transgenic animals are those that carries a foreign gene which has been deliberately injected into its genome.
Q76) Following are all breeds of cows EXCEPT A) Jersey B) Nagpuri C) Sahiwal D) Sindhi
Correct option: B Explanation: Nagpuri is not a breed of cow. It is a breed of buffalo which originates in Maharashtra.
Q75) Which are the phagocytic cells from given diagram? A) I and V B) I and III C) I and IV D) I and II
Correct option: A Explanation: I and V are the phagocytic cells. Phagocytic cells are the cells which have the capacity to ingest foreign particles.
Q74) The first vaccine produced by Edward Jenner, was for protection against A) Hepatitis B) Influenza C) Chicken pox D) Small pox
Correct option: D Explanation: The first vaccine produced by Edward Jenner, was for protection against small pox. The vaccine was developed in 1796. The vaccine lead to global eradication of small...
Q73) How many pairs of sympathetic ganglia are present in ANS? A) 10 B) 12 C) 22 D) 31
Correct option: C Explanation: 22 pairs of sympathetic ganglia are present in ANS. There are 22 pairs of ganglia: 3 in cervical region, 11 in thoracic region, 4 in lumbar region and 4 in the sacral...
Q71) Morula formed at the end of cleavage is ______ celled. A) 14 B) 16 C) 18 D) 20
Correct option: B Explanation: Morula formed at the end of cleavage is 16 celled. The zygote divides mitotically by cleavage process. Cleavage continues till a solid ball of cells called as morula...
Q70) Serotonin and Melatonin are hormones, secreted by A) Pancreas B) Pineal body C) Pituitary gland D) Thymus
Correct option: B Explanation: Pineal body secretes Serotonin and Melatonin hormones. Pineal gland is an endocrine gland. The hormone produced by it modulated the pattern of the sleep.
Q69) Which of the following options are CORRECT ? 1. Heroin – Stimulant 2. Marijuana – Cardiovascular 3. Cocaine – Hallucinations 4. Morphine – Sedative A) 1, 2 and 3 B) 1, 3 and 4 C) 2, 3 and 4 D) 1, 2 and 4
Correct option: C Explanation: . Marijuana – Cardiovascular: marijuana smoking increases the risk of cardiovascular diseases. Cocaine – Hallucinations: excessive usage of cocaine causes...
Q68) The first fossil of Australopithecus was discovered in A) Olduvai Gorge, Tanzania B) Fayum deposits of Egypt C) Siwalik hills in India D) Taung in South Africa
Correct option: D Explanation: The first fossil of Australopithecus was discovered in Taung in South Africa. The fossil was of an immature apelike individual.
Q67) Find the odd one out, with respect to X-linkage. A) Haemophilia B) Myopia C) Nephritis D) Night blindness
Correct option: C Explanation: Nephritis is the odd one with respect to X-linkage.