Solution 8: The following processes are involved in the extraction of zinc from zinc blende: (i) Concentration: Zinc blende ore is crushed and the concentration done by froth- floatation process....
Write chemical reactions taking place in the extraction of zinc from zinc blende.
Solution 8: The following processes are involved in the extraction of zinc from zinc blende: (i) Concentration: Zinc blende ore is crushed and the concentration done by froth- floatation process....
Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
Solution 7: In the blast furnace reduction of iron oxides take place at different temperature ranges as shown below. At $500-800 \mathrm{~K}$ $$ \begin{array}{l} 3 \mathrm{Fe}_{2}...
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present?
Solution The common elements present in the anode mud are antimony, selenium, tellurium, silver, gold and platinum. These elements settle down under anode as anode mud because they are less reactive...
Out of 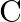 and
and 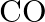 which is a better reducing agent at 673K?
which is a better reducing agent at 673K?
Solution 5: This can be explained thermodynamically, taking entropy and free energy changes into account (a) $C(s)+O_{2}(g) \rightarrow C O_{2}(g)$ (b) $2...
Explain:
(i) Zone refining
(ii) Column chromatography
Solution 4: (i) Zone refining: This method is used for production of semiconductors and other metals of very high purity, e.g., $\mathrm{Ge}, \mathrm{Si}, \mathrm{B}, \mathrm{Ca}$ and $\mathrm{In}$....
Explain:
(i) Zone refining
(ii) Column chromatography
Solution 4: (i) Zone refining: This method is used for production of semiconductors and other metals of very high purity, e.g., $\mathrm{Ge}, \mathrm{Si}, \mathrm{B}, \mathrm{Ca}$ and $\mathrm{In}$....
Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
Solution 3: $\Delta_{f} G^{o}$ of $\mathrm{Cu}_{2} \mathrm{~S}$ is more negative than $\Delta_{f} G^{\circ}$ of $\mathrm{CS}_{2} \mathrm{H}_{2} S$. So $C_{u} 2 S$ can not be reduced by carbon or...
What is the role of depressant in froth-floatation process?
Solution 2: The role of depressant is to prevent one type of sulphide ore particles from forming the froth with air bubbles. $\mathrm{NaCN}$ is used as a depressant to separate lead sulphide (PbS)...
Copper can be extracted by hydrometallurgy but not zinc. Explain.
Solution Copper can be extracted by hydrometallurgy but not zinc, this is because $E_{Z n^{2}+Z n}^{o}=-0.76 \mathrm{~V}$ lower than that of $E_{\mathrm{Cu}^{2+} / C i}^{o}=-0.34 \mathrm{~V}$ Hence,...
Is it true that under certain conditions, Mg can reduce 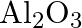 and
and 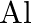 can reduce
can reduce 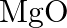 ? What are those conditions?
? What are those conditions?
Solution Yes, below $1350^{\circ} \mathrm{C}, \mathrm{Mg}$ can reduce $\mathrm{Al}_{2} \mathrm{O}_{3}$ and above $1350^{\circ} \mathrm{C}, \mathrm{Al}$ can reduce $\mathrm{MgO}$. This can be...
The reaction, 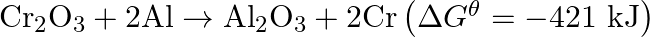 is thermodynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
is thermodynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
Solution This is explained on the basis of $\mathrm{Keq}$, the equilibrium constant. In the given redox reaction, all reactants and products are solids at room temperature, so, there is no...
What is the significance of leaching in the extraction of aluminium?
Solution 2: Aluminium contains silica $\left(\mathrm{SiO}_{2}\right)$, iron oxide $\left(\mathrm{Fe}_{2} \mathrm{O}_{3}\right)$ and titanium oxide $\left(\mathrm{TiO}_{4}\right)$ as impurities....
Which of the ores mentioned can be concentrated by magnetic separation method?
Solution Ores which are magnetic in nature can be separated from non-magnetic gangue particles by magnetic separation method. For ex: ores of iron such as haemetite $\left(\mathrm{Fe} 2...
Specify the oxidation numbers of the metals in the following coordination entities:
(i) $\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)(\mathrm{CN})(\mathrm{en})_{2}\right]^{2+}$ Ans: Let us assume that the coordination number of Co is X. Therefore, we can write: $$...
What is meant by unidentate, bidentate and ambidentate ligands? Give two examples for each.
Ans: These are explained below: (i) Unidentate ligand Ligands with only one donor site are called unidentate ligands. For example, $\mathrm{Cl}$ and $\mathrm{NH}_{3}$ are unidentate ligands. (ii)...
Specify the oxidation numbers of the metals in the following coordination entities: (i) ![Rendered by QuickLaTeX.com \left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)(\mathrm{CN})(\mathrm{en})_{2}\right]^{2+}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-5830aca5c4a498a25851f5ca5b2a9534_l3.png)
Ans: Let us assume that the coordination number of Co is X. Therefore, we can write: $$ \begin{array}{l} x+0+(-1)+2(0)=+2 \\ x-1=+2 \\ x=+3 \end{array} $$ So, the coordination number of cobalt is...
What is meant by unidentate, bidentate and ambidentate ligands? Give two examples for each.
Ans: These are explained below: (i) Unidentate ligand Ligands with only one donor site are called unidentate ligands. For example, $\mathrm{Cl}$ and $\mathrm{NH}_{3}$ are unidentate ligands. (ii)...
Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Ans: All of them are explained below: (i) Coordination entity A central metal atom or anions connected to a set number of ions or molecules known as ligands comprises a coordination entity. For...
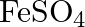 solution mixed with
solution mixed with 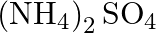 , solution in
, solution in 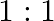 molar ratio gives the test of
molar ratio gives the test of 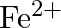 ion but
ion but 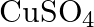 solution mixed with aqueous ammonia in
solution mixed with aqueous ammonia in 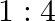 molar ratio does not give the test of
molar ratio does not give the test of 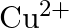 ion. Explain. why?
ion. Explain. why?
Ans: Let us see the reactions happening in both the cases. $$ \begin{array}{l} \left(\mathrm{NH}_{4}\right)_{2} \mathrm{SO}_{4}+\mathrm{FeSO}_{4}+6 \mathrm{H}_{2} \mathrm{O} \rightarrow...
Explain the bonding in coordination compounds in terms of Werner’s postulates.
Ans: Werner's theory is the first theory to explain the nature of bonding in coordination compounds. The main postulates of this theory are: (i) Two types of valencies, primary and secondary...
Calculate the overall complex dissociation equilibrium constant for the 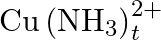 ion, given that
ion, given that 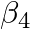 for this complex is
for this complex is 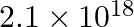 .
.
Ans: We are given the overall stability constant $\left(\beta_{s}\right)=2.1 \times 10^{13}$. The overall complex dissociation equilibrium constant is the reciprocal of the overall stability...
Write the formulas for the following coordination compounds: (i) Tetraamminediaquacobalt (III) chloride
Ans: The formula of Tetraamminediaquacobalt (III) chloride is $$ \left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)\left(\mathrm{NH}_{3}\right)_{4}\right] \mathrm{Cl}_{3} $$ (ii) Potassium...
A solution of glucose in water is labelled as 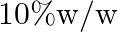 , that would be the molality and mole fraction of each component in the solution? If the density of solution is
, that would be the molality and mole fraction of each component in the solution? If the density of solution is 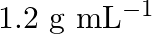 then what shall be the molarity of the
then what shall be the molarity of the
Solution 10 percent $w / w$ solution of glucose in water means $10 g$ glucose and $90 \mathrm{~g}$ of water. $10 \mathrm{~g}$ of glucose $=\frac{10}{180}=0.0555$ moles And $90 \mathrm{~g}$ of...
Density of solution 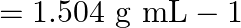 given Volume of
given Volume of
solution $-\frac{\text { mass }}{\text { density }}=\frac{100}{1.504}=66.5 \mathrm{~mL}$ Molarity of solution $$ \begin{array}{l} =\frac{\text { moles of sloution } \times 1000}{\text { Volume of...
Concentrated nitric acid used in laboratory work is 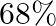 nitric acid by mass in aqueous solution. What should be the molarity of such a sample of the acid if the density of the solution is
nitric acid by mass in aqueous solution. What should be the molarity of such a sample of the acid if the density of the solution is 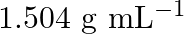 ?
?
Solution 4: $68 \%$ nitric acid by mass means that $68 \mathrm{~g}$ mass of nitric acid is dissolved in $100 \mathrm{~g}$ mass the solution. Molar mass of $\mathrm{HNO}_{3}=63 \mathrm{~g}...
Define the following terms:
(i) Mole fraction
(ii) Molality
(iii) Molarity
Solution 3: (i) Mole Fraction: It is defined as the ratio of the number of moles of the solute to the total number of moles in the solution. If $\mathrm{A}$ is the number of moles of solute...
Give an example of a solid solution in which the solute is a gas.
Solution Solution of hydrogen in palladium and dissolved gases in minerals
Calculate the osmotic pressure in pascals exerted by solution prepared by dissolving 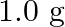 of polymer of molar mass 185,000 in
of polymer of molar mass 185,000 in 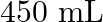 of water at
of water at 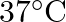 .
.
Solution $$ \begin{array}{l} \text { Given } \mathrm{V}=450 \mathrm{~mL}=0.45 \mathrm{~L} \\ =37^{\circ} \mathrm{C}=310 \mathrm{~K} \\ \mathrm{R}=8.314 \mathrm{kPaL} \mathrm{k}^{-1}...
Boiling point of water at 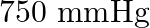 is
is 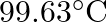 . How much sucrose is to be added to
. How much sucrose is to be added to 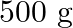 of water such that it boils at
of water such that it boils at 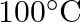 .
.
Solution Given $\Delta \mathrm{Tb}=100-99.63=3.37^{\circ}$ Mass of water w1 $=500 \mathrm{~g}$ Molar mass of water, $\mathrm{M}_{1}=18 \mathrm{~g} \mathrm{~mol}^{-1}$ Molar mass of sucrose,...
How can you determine the atomic mass of an unknown metal if you know its density and the dimension of its unit cell? Explain.
Ans: By knowing the density of an unknown metal and the dimension of its unit cell, the atomic mass of the metal can be determined. Let 'a' be the edge length of a unit cell of a crystal, 'd' be the...
What is meant by the term coordination number?
Ans: The number of nearest neighbors of any constituent particle present in the crystal lattice is called its coordination number.
How you distinguish between the following pairs of terms:
(i) Hexagonal close-packing and cubic close-packing? Ans: A 2-d hexagonal close-packing contains two types of triangular voids (a and b) as shown in figure 1. Let us call this 2-D structure as layer...
‘Stability of crystal is reflected in the magnitude of its melting point’. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the intermolecular forces between these molecules?
Ans: Higher the melting point, greater are the intermolecular forces of attraction between the atoms of a molecule and greater is the stability of that molecule. A substance with higher melting...
Niobium crystallises in body-centered cubic structure. If density is 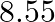
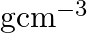 . Calculate the atomic radius of niobium using its atomic mass
. Calculate the atomic radius of niobium using its atomic mass 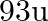 .
.
Ans: It is given that the density of niobium, $\mathrm{d}=8.55 \mathrm{gcm}^{-3}$ Atomic mass, $M=93 \mathrm{gmol}^{-1}$ As the lattice is bcc type, the number of atoms per unit cell, $z=2$ Let the...
A cubic solid is made of two elements  and
and  Atoms of
Atoms of 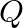 are at the corners of the cube and
are at the corners of the cube and  at the body-centre. What is the formula of the compound? What are the coordination numbers of
at the body-centre. What is the formula of the compound? What are the coordination numbers of  and
and 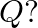
Ans: It is given that the atoms of $\mathrm{Q}$ are present at the corners of the cube. Therefore, number of atoms of $Q$ in one unit cell $=8 \times(1 / 8)=1$ It is also given that the atoms of...
Silver crystallises in the fcc lattice. If edge length of the cell is 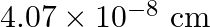 and density is
and density is 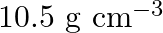 , calculate the atomic mass of silver.
, calculate the atomic mass of silver.
Ans: It is given that the edge length, $\mathrm{a}=4.07 \times 10^{-8} \mathrm{~cm}$ and density is $\mathrm{d}=$ $10.5 \mathrm{~g} \mathrm{~cm}^{3}$ As the lattice is foc type, the number of atoms...
Stability of crystal is reflected in the magnitude of its melting point’. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the intermolecular forces between these molecules?
Ans: Higher the melting point, greater are the intermolecular forces of attraction between the atoms of a molecule and greater is the stability of that molecule. A substance with higher melting...
Niobium crystallises in body-centered cubic structure. If density is 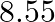
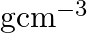 . Calculate the atomic radius of niobium using its atomic mass
. Calculate the atomic radius of niobium using its atomic mass 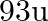 .
.
Ans: It is given that the density of niobium, $\mathrm{d}=8.55 \mathrm{gcm}^{-3}$ Atomic mass, $M=93 \mathrm{gmol}^{-1}$ As the lattice is bcc type, the number of atoms per unit cell, $z=2$ Let the...
A cubic solid is made of two elements  and
and 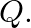 Atoms of
Atoms of 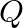 are at the corners of the cube and
are at the corners of the cube and  at the body-centre. What is the formula of the compound? What are the coordination numbers of
at the body-centre. What is the formula of the compound? What are the coordination numbers of  and
and 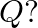
Ans: It is given that the atoms of $\mathrm{Q}$ are present at the corners of the cube. Therefore, number of atoms of $Q$ in one unit cell $=8 \times(1 / 8)=1$ It is also given that the atoms of...
Niobium crystallises in body-centered cubic structure. If density is 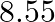
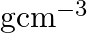 . Calculate the atomic radius of niobium using its atomic mass
. Calculate the atomic radius of niobium using its atomic mass 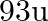 .
.
Ans: It is given that the density of niobium, $\mathrm{d}=8.55 \mathrm{gcm}^{-3}$ Atomic mass, $M=93 \mathrm{gmol}^{-1}$ As the lattice is bcc type, the number of atoms per unit cell, $z=2$
A cubic solid is made of two elements  and
and 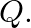 Atoms of
Atoms of 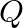 are at the corners of the cube and
are at the corners of the cube and  at the body-centre. What is the formula of the compound? What are the coordination numbers of
at the body-centre. What is the formula of the compound? What are the coordination numbers of  and
and 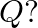
Ans: It is given that the atoms of $\mathrm{Q}$ are present at the corners of the cube. Therefore, number of atoms of $Q$ in one unit cell $=8 \times(1 / 8)=1$ It is also given that the atoms of...
A cubic solid is made of two elements  and
and 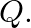 Atoms of
Atoms of 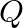 are at the corners of the cube and
are at the corners of the cube and  at the body-centre. What is the formula of the compound? What are the coordination numbers of
at the body-centre. What is the formula of the compound? What are the coordination numbers of  and
and 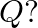
Ans: It is given that the atoms of $\mathrm{Q}$ are present at the corners of the cube. Therefore, number of atoms of $Q$ in one unit cell $=8 \times(1 / 8)=1$ It is also given that the atoms of...
Silver crystallises in the fcc lattice. If edge length of the cell is 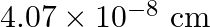 and density is
and density is 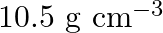 , calculate the atomic mass of silver.
, calculate the atomic mass of silver.
Ans: It is given that the edge length, $\mathrm{a}=4.07 \times 10^{-8} \mathrm{~cm}$ and density is $\mathrm{d}=$ $10.5 \mathrm{~g} \mathrm{~cm}^{3}$ As the lattice is foc type, the number of atoms...
Copper crystallises into a fcc lattice with edge length 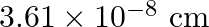 . Show that the calculated density is in agreement with its measured value of
. Show that the calculated density is in agreement with its measured value of 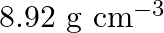
Ans: Edge length, $a=3.61 \times 10^{-6} \mathrm{~cm}$ As the lattice is fcc type, the number of atoms per unit cell, $z=4$ Atomic mass, $\mathrm{M}=63.5 \mathrm{~g} \mathrm{~mol}^{-1}$ We also know...
What is a semiconductor? Describe the two main types of semiconductors and contrast their conduction mechanism.
Ans: Semiconductors are substances having conductance in the intermediate range $10^{-6}$ to $10^{4}$ ohm $^{-1} \mathrm{~m}^{-1}$. The two main types of semiconductors are: (i) n-type semiconductor...
Classify each of the following as being either a 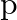 -type or an
-type or an 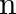 -type semiconductor.
-type semiconductor.
(i) Ge doped with In Ans: Ge (a group 14 element) is doped with In (a group 13 element). Therefore, a hole will be created and the semiconductor generated will be a p-type semiconductor. (ii) B...
Ferric oxide crystallises in a hexagonal close-packed array of oxide ions with two out of every three octahedral holes occupied by ferric ions. Derive the formula of the ferric oxide.
Ans: Let the number of oxide $\left(\mathrm{O}^{2-}\right)$ ions be $\mathrm{x}$. So, number of octahedral voids $=x$ It is given that two out of every three octahedral holes are occupied by ferric...
If 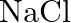 is doped with
is doped with 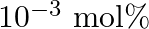 of
of 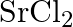 , what is the concentration of cation vacancies?
, what is the concentration of cation vacancies?
Ans: It is given that $\mathrm{NaCl}$ is doped with $10^{3} \mathrm{~mol} \%$ of $\mathrm{SrCl}_{2}$. This means that $100 \mathrm{~mol}$ of $\mathrm{NaCl}$ is doped with $10^{3} \mathrm{~mol}$ of...
Aluminium crystallises in a cubic close-packed structure. Its metallic radius is 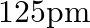 .
.
(i) What is the length of the side of the unit cell? Ans: For cubic close-packed structure: $$ \begin{array}{l} \mathrm{a}=2 \sqrt{2} \mathrm{r} \\ \Rightarrow 2 \sqrt{2}=125 \mathrm{pm} \\ =353.55...
If 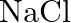 is doped with
is doped with 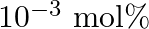 of
of 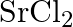 , what is the concentration of cation vacancies?
, what is the concentration of cation vacancies?
Ans: It is given that $\mathrm{NaCl}$ is doped with $10^{3} \mathrm{~mol} \%$ of $\mathrm{SrCl}_{2}$. This means that $100 \mathrm{~mol}$ of $\mathrm{NaCl}$ is doped with $10^{3} \mathrm{~mol}$ of...
Aluminium crystallises in a cubic close-packed structure. Its metallic radius is 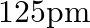 .
.
(i) What is the length of the side of the unit cell? Ans: For cubic close-packed structure: $$ \begin{array}{l} \mathrm{a}=2 \sqrt{2} \mathrm{r} \\ \Rightarrow 2 \sqrt{2}=125 \mathrm{pm} \\ =353.55...
Refractive index of a solid is observed to have the same value along all directions. Comment on the nature of this solid. Would it show cleavage property?
Ans: As isotropic solid has the same value of physical properties when measured along different directions. Therefore, the given solid, having the same value of refractive index along all...
Why is glass considered a super cooled liquid?
Ans: Similar to liquids, glass has a tendency to flow, though very slowly. Therefore, glass is considered as a super cooled liquid. This is the reason that glass windows and doors are slightly...
Why do solids have a definite volume?
Ans: The intermolecular forces of attraction that are present in solids are very strong, The constituent particles of solids have fixed positions i.e., they are rigid. Hence, solids have a definite...
Why are solids rigid?
Ans: The intermolecular forces of attraction that are present in solids are very strong. The constituent particles of solids cannot move from their positions i.e., they have fixed positions....
$ Solid A is a very hard electrical insulator in solid as well as in molten state and melts at extremely high temperature. What type of solid is it?
Ans: The given properties are the properties of a covalent or network solid. Therefore, the given solid is a covalent or network solid. Examples of such solids include diamond ( $\mathrm{C}$ ) and...
Classify the following solids in different categories based on the nature of intermolecular forces operating in them: Potassium sulphate, tin, benzene, urea, ammonia, water, zinc sulphide, graphite, rubidium, argon, silicon carbide.
Ans: Potassium sulphate $\rightarrow$ ionic solid Tin Metallic $\rightarrow$ solid Benzene $\rightarrow$ Molecular (non-polar) solid Urea $\rightarrow$ Polar molecular solid Ammonia $\rightarrow$...
Distinguish between
(i) Hexagonal and monoclinic unit cells Ans: Hexagonal unit cell: For a hexagonal unit cell $$ \begin{array}{l} a=b \neq c \\ \text { and } \alpha=\beta= \\ y=120^{\circ} \end{array} $$ Monoclinic...
Name the parameters that characterize a unit cell.
Ans: The six parameters that characterize a unit cell are as follows. (i) Its dimensions along the three edges, $a, b$, and $c$. These edges may or may not be equal. (ii) Angles between the edges....
Give the significance of a lattice point.
Ans: The significance of a lattice point is that each lattice point represents one constituent particle of a solid which may be an atom, a molecule (group of atom), or an ion.
A compound is formed by two elements 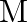 and
and 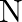 . The element
. The element 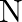 forms ccp and atoms of M occupy
forms ccp and atoms of M occupy 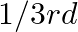 of tetrahedral voids. What is the formula of the compound?
of tetrahedral voids. What is the formula of the compound?
Ans: The ccp lattice is formed by the atoms of the element $\mathrm{N}$. Here, the number of tetrahedral voids generated is equal to twice the number of atoms of the element $\mathrm{N}$. According...
A compound forms a hexagonal close-packed structure. What is the total number of voids in 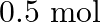 of it? How many of these are tetrahedral voids?
of it? How many of these are tetrahedral voids?
A compound is formed by two elements $\mathrm{M}$ and $\mathrm{N}$. The element $\mathrm{N}$ forms ccp and atoms of M occupy $1 / 3 r d$ of tetrahedral voids. What is the formula of the compound?
What is the two dimensional coordination number of a molecule in a square close packed layer?
Ans: In a square close-packed layer, the molecule is in contact with four of its neighbours, Therefore, the two-dimensional coordination number of a molecule in a square close packed layer is $4 .$
Explain how much portion of an atom located at
(i) corner and
(ii) bodycentre of a cubic unit cell is part of its neighbouring unit cell.
Ans: (i) An atom located at the corner of a cubic unit cell is shared by eight adjacent unit cells. Therefore, 1 / 8 th portion of the atom is shared by one unit cell. (ii) An atom located at the...
What type of stoichiometric defect is shown by:
(i) $\mathrm{ZnS}$, Ans: ZnS shows Frenkel defect. (ii) $\mathrm{AgBr}$ Ans: AgBr shows Frenkel defect as well as Schottky defect.
What type of defect can arise when a solid is heated? Which physical property is affected by it and in what way?
Ans: When a solid is heated, vacancy defects can arise. A solid crystal is said to have vacancy defects when some of the lattice sites are vacant. Vacancy defect leads to a decrease in the density...
An element with molar mass 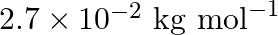 forms a cubic unit cell with an edge length
forms a cubic unit cell with an edge length 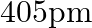 . If its density is
. If its density is 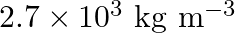 what is the nature of the cubic unit cell?
what is the nature of the cubic unit cell?
Ans: It is given that density of the element, $d=2.7 \times 10^{3} \mathrm{~kg} \mathrm{~m}^{-3}$ Molar mass, $M=2.7 \times 10^{-2} \mathrm{~kg} \mathrm{~mol}^{-1}$ Edge length, $\mathrm{a}=405...
What type of substances would make better permanent magnets, ferromagnetic or ferrimagnetic. Justify your Solution.
Ans: Ferromagnetic substances would make better permanent magnets. In solid state, the metal ions of ferromagnetic substances are grouped together into small regions. These regions are called...
A group 14 element is to be converted into n-type semiconductor by doping it with a suitable impurity. To which group should this impurity belong?
Ans: An n-type semiconductor conducts because of the presence of extra electrons. Therefore a group 14 element can be converted to n-type semiconductor by doping it with a group 15 element.
Ionic solids, which have anionic vacancies due to metal excess defect, develop colour. Explain with the help of a suitable example.
Ans: The colour develops because of the presence of electrons in the anionic sites. These electrons absorb energy from the visible part of radiation and get excited. For example, when crystals of...
Explain how vacancies are introduced in an ionic solid when a cation of higher valence is added as an impurity in it.
Ans: When a cation of higher valence is added to an ionic solid as an impurity to it, the cation of higher valence replaces more than one cation of lower valence so as to keep the crystal...
Complete the following acid-base reactions and name the products:
i. $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{NH}_{2}+\mathrm{HCl} \rightarrow$ Ans: n-propylammonium chloride ii. $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3}...
Calculate the vapour pressure of water for this solution and its relative lowering.
Solution 9: \begin{tabular}{l} \hline $\mathrm{P} \square=23.8 \mathrm{~mm} \mathrm{Hg}$ \\ $\mathrm{W} 2=50 \mathrm{~g}, \mathrm{M}_{2}($ urea $)=60 \mathrm{~g} \mathrm{~mol}^{-1}$ \\ $\mathrm{w}...
Heny’s law constant for 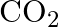 in water is
in water is 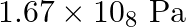 at
at 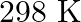 . Calculate the quantity of
. Calculate the quantity of 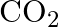 in
in 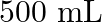 of soda water when packed under
of soda water when packed under 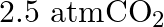 pressure at
pressure at 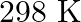 .
.
Solution 7: $$ \mathrm{KH}=1.67 \times 10_{8} \mathrm{~Pa} $$ $$ \begin{array}{l} \hline P_{A}^{o}=450 \mathrm{~mm}, P_{B}^{o}=700 \mathrm{~mm}, P_{\text {total }}=600 \mathrm{~mm} \\ \text { As...
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complexes: (i) ![Rendered by QuickLaTeX.com \mathrm{K}_{5}\left[\mathrm{Co}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{3}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-32cbfc6fdf62516d53f060567ba2033d_l3.png)
Ans: The complex will be $\left[\mathrm{Co}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{8}\right]^{3}$. Oxidation state of cobalt will be $=x-6=-3$ The oxidation state is $=+3$ Coordination number...
Discuss the nature of bonding in metal carbonyls.
Ans: The carbon metal linkages in metal carbonyls are characterized by both s and p. M-Ca bond consists of a donation into an empty metal orbital of a lone pair of electrons on the carbonyl carbon....
What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Ans: In $\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3 v}$ the oxidation state of $\mathrm{Cr}$ is in $+3$ oxidation state, which will cause the configuration of $\mathrm{Cr}$ as...
Using IUPAC norms Write the systematic names of the following:
(i) $\quad\left[\mathrm{CO}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{3}$ Ans: The IUPAC name of the compound is Hexaamminecobalt(III) chloride. (ii)...
Calculate the mass of urea (NH2CONH2) required in making 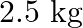 of
of 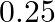 molal aqueous solution.
molal aqueous solution.
Solution 4: $0.25$ molal aqueous solution to urea means that Moles of urea $=0.25$ mole Mass of solvent $(\mathrm{NH} 2 \mathrm{CONH} 2)=60 \mathrm{~g} \mathrm{~mol}^{-1}$ $\therefore 0.25$ mole of...
Calculate the molarity of each of the following solution: (a) 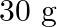 of
of 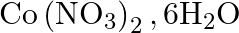 in
in 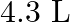 of
of
solution (b) $30 \mathrm{~mL}$ of $0.5 \mathrm{M} \mathrm{H}_{2} \mathrm{SO}_{4}$ diluted to $500 \mathrm{~mL}$. Solution 3: (a) Molar mass of $\mathrm{Co}\left(\mathrm{NO}_{3}\right)_{2}, 6...
Calculate the mole fraction of benzene in solution containing 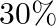 by mass in carbon tetrachloride.
by mass in carbon tetrachloride.
Solution 2: $30 \%$ by mass of $\mathrm{C} 6 \mathrm{H} 6$ in $\mathrm{CCl} 4 \Rightarrow 30 \mathrm{~g} \mathrm{C} 6 \mathrm{H} 6$ in $100 \mathrm{~g}$ solution $\therefore$ Number of moles of...
Calculate the mass percentage of benzene 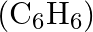 and carbon tetrachloride
and carbon tetrachloride 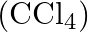 if
if 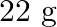 of benzene is dissolved in
of benzene is dissolved in 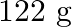 of carbon tetrachloride.
of carbon tetrachloride.
Solution Mass percentage of $$ \begin{array}{l} C_{6} H_{6}=\frac{\text { Mass of } \mathrm{C}_{6} \mathrm{H}_{6}}{\text { Total mass of the solution }} \times 100 \% \\ =\frac{\text { Mass of }...
Classify each of the following solids as ionic, metallic, molecular, network (covalent) or amorphous.
(i) Tetra phosphorus decoxide $\left(\mathrm{P}_{4} \mathrm{O}_{20}\right)$ (ii)Ammonium phosphate $\left(\mathrm{NH}_{4}\right)_{3} \mathrm{PO}_{4}$ (iii) $\mathrm{SiC}$ (iv) $\mathrm{I}_{2}$ (v)...
What makes a glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?
Ans: The arrangement of the constituent particles makes glass different from quartz. In glass, the constituent particles have short range order, but in quartz, the constituent particles have both...
Define the term ‘amorphous’ give a few examples of amorphous solids.
Ans: Amorphous solids are the solids that have their constituent particles of irregular shape and have short range order. These solids are isotropic in nature and melt over a range of temperature....
How are synthetic defergents better than soup?
Solution Soaps work in soft water. However, they are not effective in hard water. In contrast, synthetic detergents work both in soft water and hard water. Therefore, synthetic detergents are better...
What problem arises in using alitame as artificial sweetener?
Solution Alitame is a high potency sweetener. It is difficult to control the sweetness of food while using alitame as an artificial sweetener.
Name a sweetening agent used in the preparation of sweets for a diabetic patient
Solution Artificial sweetening agents such as saceharin, alitame, and aspartame can be used in preparing sweets for diabetie paticats. Question 19: What problem arises in using alitame as artificial...
What are artificial sweetening agents? Give two examples.
Solution Artificial sweetening agents are chemicals that sweeten food. However, unlike natural sweeteners, they do not add calories to our body. They do not harm the human body. Some artificial...
Why is use of aspartame limited to cold foods and drinks?
Solution Aspartame becomes unstable at cooking temperature. This is the reason why its use is limited to cold foods and drinks.
What are food preservatives?
Solution Food preservatives are chemicals that prevent food from spoilage due to microbial growth. Table salt, sugar, vegetable oil, sodium benzoate (ChHsCOONa), and salts of propanoic acid are...
What is tincture of iodine? What is its use?
Solution Tincture of iodine is a $2-3$ percent solution of wodine in alcohol - water mixture, It is applied to wounds as an antiseptic.
What are the main constituents of dettol?
Solution The main constituents of dettol are chloroxylenol and a-terpineol. CC1=CC(O)=CC(C)C1Cl CC1=CCC(C(C)(C)Br)CC1 Chatisiars a-ticyanat
Name a substance which can be used as an antiseptic as well as disinfectant.
Solution Phenol can be used as an antiseptic as well as a disinfectant. $0.2$ peroent solution of phenol is used as an antiseptic, while 1 per cent of its solution is used as a disinfectant.
Why are cimetidine and ranitidine better antacids than sodium hydrogen carbonate or magnesium or aluminium hydroxide?
Solution Cimetidine and rantidine are better antacids as they control the root eause of acidity. These drugs prevent the interaction of histamine with the receptors present in the stomach walls....
How do antiseptics differ from disinfectants? Give one example of each.
Solution Antiseptics and disinfectants are effective against micro-organisms. However, antiseptics are applied to the living tisswes such as wounds, cuts, ulcers, and diseased skin surfaces, while...
What is meant by the term ‘broad spectrum antibiotics’? Explain.
Solution 9: Antibiotics that are effective against a wide range of gram-positive and gram-negative bacteria are known as broad spectrum antibiotics. Chloramphenicol is a broud spectrum antibiotic....
Low level of noradrenaline is the cause of depression. What types of drugs are needed to cure this problem? Name two drugs
Solution \&: Anti-depressant drugs are needed to counteract the effect of depression. These drugs inhibit Enzymes catalysing the degradation of the neurotransmitter, noradrenaline. As a result, the...
While antacids and antiallergic drugs interfere with the function of histamines, why do these not interfere with the function of each other?
Solution Specific drugs affect particular receptors. Antacids and anti-allergie drugs work on different receptors. This is the reason why antacids and anti-allergic drugs do not interfere with each...
Which foroes are involved in holding the drugs to the active site of enzymes?
Solution Either of the following forces can be involved in holding drugs to the active sites of enzymes, (i) Ionic bonding (ii) Hydrogen bonding (iii) Dipole - dipole interaction (iv) van der Waals...
Define the tern chemotherapy.
Solution The use of chemicals for therapeutic effect is called chemotherapy. For example: the use of chemicals in the diagnosis, prevention, and treatment of diseases
Name the macromolecules that are chosen as drug targets.
Solution The macromolecules chosen as drug targets are carbohydrates, lipids, proteins, and nucleic acids,
Explain the term target molecules or drug targets as used in medicinal chemistry.
Solution In medicinal chemistry, drug targets refer to the key molecules involved in certain metabolic pathways that result in specific diseases, Carbohydrates, proteins, lipids, and nucleic acids...
Why do we need to classify drugs in different ways?
Solution The classification of drugs and the reasons for classification are as follows: (i) On the basis of pharmacological effect: This classification provides doctors the whole range of drugs...
Complete the following acid-base reactions and name the products:
i. $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{NH}_{2}+\mathrm{HCl} \rightarrow$ Ans: n-propylammonium chloride ii. $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3}...
Arrange the following in increasing order of their basic strength: i. 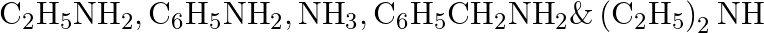
Ans: Considering the inductive effect of alkyl groups $\mathrm{NH}_{3}, \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ and $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ can be...
An organic compound with the molecular formula 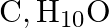 forms 2,4 -DNP derivative, reduces Tollens’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2 -benzenedicarboxylic acid. Identify the compound.
forms 2,4 -DNP derivative, reduces Tollens’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2 -benzenedicarboxylic acid. Identify the compound.
Ans: It is because the chemical $\left(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\right)$ generates a derivative of 2,4 -dnp and reduces the reagent of Tollen. The chemical must thus be an aldehyde....
Draw structures of the following derivatives.
$\mathrm{H}_{3} \mathrm{C} \longrightarrow{\mathrm{C}}_{2}^{\mathrm{H}_{2}} \mathrm{CHO}+\mathrm{H}_{3} \mathrm{C} \longrightarrow \stackrel{\mathrm{H}_{2}}{\mathrm{C}}-\mathrm{CHO} \longrightarrow...
Write the IUPAC names of the following ketones and aldehydes. Whenever possible, give also common names.
(i) $\mathrm{CH}_{3} \mathrm{CO}\left(\mathrm{CH}_{2}\right)_{4} \mathrm{CH}_{3}$ Ans: The IUPAC name of the given compound is Heptan-2-one and its common name is Methyl n-pentyl ketone. (ii)...
Draw the structures of the following compounds:
(i) 3-Methylbutanal Ans: The structure of 3-Methylbutanal is given below: CC(C)CC=O (ii) p-Nitropropiophenone Ans: The structure of p-Nitropropiophenone is given below: (viii) Hex-2-en-4-ynoic acid...
Name the following compounds according to IUPAC system of nomenclature: (i) 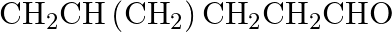
Ans: The IUPAC name of the given compound is 4-Methylpentanal, (ii) $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COCH}\left(\mathrm{C}_{2} \mathrm{H}_{5}\right) \mathrm{CH}_{2} \mathrm{CH}_{2}...
Following type of non-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic parts in the molecule. Ideatify the functional group (s) present in the molecule.  to 109
to 109
Solution 5: Functional groups present in the molecule are: Hy trophobic part (i) Ether, and (ii) primary alcoholic group wime yodansu cion 10 "odididu Lice onun: r.toning $\mathrm{R}-\mathrm{C}=$...
Write the chernical equation for preparing sodium soap from glyceryl oleate and glyceryl palmitate. Structural formulae of these compounds are given below. (i)  Glyceryl palmitate (ii)
Glyceryl palmitate (ii) 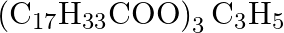 -Glyceryl oleate
-Glyceryl oleate
Solution Sodiam palmoie (Soup) Glyiery palmitate Olyserol (ii) Glyceryl oleses Görerd
Why do we require artificial sweetening agents?
Solution A large number of pcople are suffering from diseases such as diabetes and obesity. These people cannot take normal sugar i.e., sucrose as it is hamful for them. Therefore, artificial...
With reference to which classification has the statement, ‘ranitidine is an antacid'” been given?
Solution The given statement refers to the classification of pharmacological effects of the drug. This is because any drug that is used to counteract the effects of excess acid in the stomach is...
Sleeping pills are recommended by doctors to the patients suffering from sleeplessness but it is not advisable to take its doses without consultation with the doctor, Why?
Solation Most drugs when taken in doses higher than recommended may cause harmful effects and sometimes, may even lead to death. Hence, a doctor should always be consulted before taking any medicine
Explain the cleansing action of soaps
Solution Soap molecules form micelles around an oil droplet (dirt) in such a way that the hydrophobic parts of the stearate ions attach themselves to the oil droplet and the hydrophilic parts...
Can you use soaps and synthetic detergents to check the hardness of water?
Solution Soaps get precipitated in hard water, but not in sott water. Therefore, soups can be used for checking the hardness of water- However, synthetic detergents do not get procipitated cither in...
Why do soaps not work in hard water?
Solution Soaps are sodium or potassium salts of long-chain fatty acids. Hard water contains calcium and magnesium ions. When soaps are dissolved in hard water, these ions displace sodium or...
What are biodegradable and non-biodegradable detergeats? Give one example of each.
Solution Detergents that can be degraded by bicteria are called biodegradable detergents. Such detergents have straight hydrocarbon chains, For example: sodium lauryl sulphate Detergents that cannot...
Explain the following terms with suitable examples.
(i) Cationic detergents
(ii) Anionic detergents and
(iii) Non-ionic detergents
Solution 21: (i) Cationic detergent Cationic detergents are quatemary ammonium salts of acetates, chlorides, or bromides. These are called cationic detergents because the cationic part of these...
How are synthetic defergents better than soup?
Solution Soaps work in soft water. However, they are not effective in hard water. In contrast, synthetic detergents work both in soft water and hard water. Therefore, synthetic detergents are better...
What problem arises in using alitame as artificial sweetener?
Solution Alitame is a high potency sweetener. It is difficult to control the sweetness of food while using alitame as an artificial sweetener.
Name a sweetening agent used in the preparation of sweets for a diabetic patient
Solution Artificial sweetening agents such as saceharin, alitame, and aspartame can be used in preparing sweets for diabetie paticats.
Why is use of aspartame limited to cold foods and drinks?
Solution Aspartame becomes unstable at cooking temperature. This is the reason why its use is limited to cold foods and drinks.
What are food preservatives?
Soluthon Food preservatives are chemicals that prevent food from spoilage due to microbial growth. Table salt, sugar, vegetable oil, sodium benzoate (ChHsCOONa), and salts of propanoic acid are...
What is tincture of iodine? What is its use?
Solution Tincture of iodine is a $2-3$ percent solution of wodine in alcohol - water mixture, It is applied to wounds as an antiseptic.
What are the main constituents of dettol?
Solution 13: The main constituents of dettol are chloroxylenol and a-terpineol. CC1=CC(O)=CC(C)C1Cl CC1=CCC(C(C)(C)Br)CC1 Chatisiars a-ticyanat
Name a substance which can be used as an antiseptic as well as disinfectant.
Solution Phenol can be used as an antiseptic as well as a disinfectant. $0.2$ peroent solution of phenol is used as an antiseptic, while 1 per cent of its solution is used as a disinfectant.
Why are cimetidine and ranitidine better antacids than sodium hydrogen carbonate or magnesium or aluminium hydroxide?
Solution Antacids such as sodium hydrogen carbonate, magnesium hydroxide, and aluminium hydroxide work by neutralising the excess hydrochloric acid present in the stomach. However, the root cause...
How do antiseptics differ from disinfectants? Give one example of each.
Solution Antiseptics and disinfectants are effective against micro-organisms. However, antiseptics are applied to the living tisswes such as wounds, cuts, ulcers, and diseased skin surfaces, while...
What is meant by the term ‘broad spectrum antibiotics’? Explain.
Solution 9: Antibiotics that are effective against a wide range of gram-positive and gram-negative bacteria are known as broad spectrum antibiotics. Chloramphenicol is a broud spectrum antibiotic....
What is meant by the term ‘broad spectrum antibiotics’? Explain.
Solution Antibiotics that are effective against a wide range of gram-positive and gram-negative bacteria are known as broad spectrum antibiotics. Chloramphenicol is a broud spectrum antibiotic....
Low level of noradrenaline is the cause of depression. What types of drugs are needed to cure this problem? Name two drugs
Solution Anti-depressant drugs are needed to counteract the effect of depression. These drugs inhibit Enzymes catalysing the degradation of the neurotransmitter, noradrenaline. As a result, the...
While antacids and antiallergic drugs interfere with the function of histamines, why do these not interfere with the function of each other?
Solution Specific drugs affect particular receptors. Antacids and anti-allergie drugs work on different receptors. This is the reason why antacids and anti-allergic drugs do not interfere with each...
Which foroes are involved in holding the drugs to the active site of enzymes?
Solution Either of the following forces can be involved in holding drugs to the active sites of enzymes, (i) Ionic bonding (ii) Hydrogen bonding (iii) Dipole - dipole interaction (iv) van der Waals...
Define the tern chemotherapy.
Solution The use of chemicals for therapeutic effect is called chemotherapy. For example: the use of chemicals in the diagnosis, prevention, and treatment of diseases
Why should not medicines be taken without consulting doctors?
Solution 4: A medicine can bind to more than one receptor site. Thus, a medicine may be toxic for some receptor sites. Further, in most cases, medicines cause harmful effects when taken in higher,...
Name the macromolecules that are chosen as drug targets.
Solution 3; The macromolecules chosen as drug targets are carbohydrates, lipids, proteins, and nucleic acids,
Explain the term target molecules or drug targets as used in medicinal chemistry.
Solation In medicinal chemistry, drug targets refer to the key molecules involved in certain metabolic pathways that result in specific diseases, Carbohydrates, proteins, lipids, and nucleic acids...
Why do we need to classify drugs in different ways?
Solution 1: The classification of drugs and the reasons for classification are as follows: (i) On the basis of pharmacological effect: This classification provides doctors the whole range of drugs...
What products would be formed when a nucleotide from DNA containing thymine is hydrolyzed?
Ans: The hydrolysis of a nucleotide of DNA having thymine as its base give thymine $\beta$-D-2 deoxyribose and phosphoric acid as products.
Wriny cannot vitamin C be stored in our body?
Ans: The water-soluble compounds cannot retain in human body due to constant excretion through urine. The vitamin $\mathrm{C}$ is water soluble component in our body and thus, cannot be stored,
Where does the water present in the egg go after boiling the egg?
Ans: When we boil the egg, the proteins present within them gets denatured and thus, goes under coagulation. The excess water present is then absorbed by the coagulated protein through $\mathrm{H}$...
What do you understand by the term glycosidic linkage?
Ans: The linkage formed between two monosaccharide units through an oxygen atom by the loss of a water molecule is known as glycosidic linkage. For example: Sucrose molecule has a glycosidic linkage...
Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose,
Ans: The classification is given as; Monosaccharides: Ribose, 2-deoxyribose, galactose and fructose, Disaccharides: Maltose and lactose.
Write two main functions of carbohydrates in plants.
Ans: The two main functions of carbohydrates (polysaccharides) in plants are: - Starch serves as storage molecules. - Cellulose is used to build the cell wall.
What are reducing sugars?
Ans: The carbohydrates that reduce Fehling's solution and Tollen's reagent are known as reducing sugars. All the monosaccharide and disaccharides are reducing sugars, except for sucrose.
What are monosaccharides?
Ans: Monosaccharides are the most basic units of the biomolecules. They cannot be hydrolyzed further to give simpler units. They are then classified on the basis of; - Number of C atoms: trioses,...
What are essential and non-essential amino acids? Give two examples of each type.
Ans: There are two types of amino acids in the human body: Essential amino acids: - They are required by the body but cannot be synthesized within. - These must be taken up via food. - For example,...
Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Ans: The reactions of D-glucose which cannot be explained by its open structure are; - $2,4-\mathrm{DNP}$ test, Schiff"s test and reaction with $\mathrm{NaHSO}_{4}$ to form hydrogen sulphite as...
How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Ans: Vitamins are classified on the basis of their solubility in water or fat as follows; - Vitamins such as A, D, E and K are soluble in fats and oils but not in Water. - $B$ group vitamins...
What is the effect of denaturation on the structure of proteins?
Ans: Denaturation results in unfolding of globules and uncoiling of helixes. During this process, secondary and tertiary proteins are destroyed but the primary ones remain unaltered. Sometimes,...
What are enzymes?
Ans: The proteins that catalyze the biological reactions or biological catalysts are known as enzymes. They are very specific in nature and catalyze only a particular reaction for a particular...
When RNA is hydrolyzed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
Ans: Considering a DNA molecule; it has double stranded structure in which adenine always pairs up with thymine and cytosine always pairs up with guanine through $\mathrm{H}$ - bonding. Thus, when...
What products would be formed when a nucleotide from DNA containing thymine is hydrolyzed?
Ans: The hydrolysis of a nucleotide of DNA having thymine as its base give thymine $\beta$-D-2 deoxyribose and phosphoric acid as products.
Wriny cannot vitamin C be stored in our body?
Ans: The water-soluble compounds cannot retain in human body due to constant excretion through urine. The vitamin $\mathrm{C}$ is water soluble component in our body and thus, cannot be stored,
Where does the water present in the egg go after boiling the egg?
Ans: When we boil the egg, the proteins present within them gets denatured and thus, goes under coagulation. The excess water present is then absorbed by the coagulated protein through $\mathrm{H}$...
Coupling reaction
Ans: In this reaction, arenediazonium salt reacts with aromatic amino compounds (in acidic medium) or a phenol (in alkaline medium) to form brightly coloured azo compounds. The reaction generally...
‘Hofmann’s bromamide reaction
'Hofmann's bromamide reaction Ans: When an amide is treated with bromine in alkali solution, it is converted to a primary amine that has one carbon atom less than the starting amide. This reaction...
Diazotization
Ans: The process of conversion of a primary aromatic amino compound into a diazonium salt, is known as diazotization. This process is carried out by adding. an aqueous solution of sodium nitrite to...
![Rendered by QuickLaTeX.com \mathrm{R}_{3} \mathrm{~N}+\mathrm{H}^{+} \rightarrow\left[\mathrm{R}_{3} \mathrm{NH}\right]^{+}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-5ac56813d343718c0f549e7248c3043f_l3.png) 7. Write a short note on the following. i. Carbylamine reaction
7. Write a short note on the following. i. Carbylamine reaction
Ans: Both aliphatic and aromatic primary amines when warmed with chloroform and an alcoholic solution of $\mathrm{KOH}$, produces isocyanides or carbylamines which have a very unpleasant odour. This...
Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reactions involved.
Ans: The three types of amines can be distinguished by the Heinsberg test. In this test, the amine is shaken with benzene sulphonyl chloride $\left(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{SO}_{2}...
Nitromethane into dimethylamine
Ans: $$ \mathrm{CH}_{3} \mathrm{NO}_{2} \stackrel{\text { snilhe }}{\longrightarrow} \mathrm{CH}_{3} \mathrm{NH}_{2} \stackrel{\text { oHCl. KOHs }}{\rightarrow} \mathrm{CH}_{3} \mathrm{NC}...
Methanamine into ethanamine
Ans: $$ \begin{array}{l} \mathrm{CH}_{3} \mathrm{NH}_{2} \stackrel{\mathrm{HONO}}{-\mathrm{N}_{2}-\mathrm{H}_{4} \mathrm{O}} \longrightarrow \mathrm{CH}_{3} \mathrm{OH}...
Ethanoic acid into propanoic acid
Ans: $$ \mathrm{CH}_{3} \mathrm{COOH} \stackrel{\mathrm{LiAl}_{4}}{\rightarrow \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH}} \underset{\mathrm{ONPH}_{1}}{\mathrm{P}+\mathrm{H}_{3}} \rightarrow...
Give one chemical test to distinguish between the following pairs of compounds:
i. Methylamine and dimethylamine Ans: Methylamine and methylamine can be distinguished by carbylamine test. $\mathrm{CH}_{3} \mathrm{NH}_{2}+\mathrm{CHCl}_{3}+3 \mathrm{KOH}...
Arrange the following in increasing order of their basic strength:
i. $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}, \mathrm{NH}_{3}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}...
Glucose is an aldose sugar but it does not react with sodium hydrogen sulphite. Give a reason.
Ans. An aldehyde group is present in glucose. It, on the other hand, does not react with sodium hydrogen sulphite to generate bisulphite addition products. This is because this reaction takes place...
Give reason: Amylase present in the saliva becomes inactive in the stomach.
Ans. As hydrochloric acid is present in the stomach lining; this causes the stomach to have a low $\mathrm{pH}$ value. The $\mathrm{pH}$ of the stomach is about $1-2$ which is extremely acidic, this...
Why sucrose is called invert sugar?
Ans. When sucrose is hydrolyzed, the sign of rotation changes from dextro $(+)$ to laevo $(-)$, and the result is known as invert sugar.
Sodium hydrogen carbonate and ranitidine are used as antacids. Which one is a better choice? Why?
Ans: Antacids such as sodium hydrogen carbonate, magnesium hydroxide, and aluminium hydroxide function by neutralising excess hydrochloric acid in the stomach. However, the underlying reason for...
Low level of noradrenaline is the cause of depression. Suggest drugs to cure this problem?
Ans: Depression is caused by a lack of noradrenaline. Antidepressant medications are required to treat this condition. They stop noradrenaline breakdown enzymes from working. As a result,...
Detergents containing unbranched chains are more preferable than those containing branched chains. State the reason.
Ans: Detergents containing branched chain hydrocarbons are non-biodegradable because bacteria have difficulty degrading them. As a result, straight chain hydrocarbon detergents are favoured over...
Sodium and Potassium soaps are only used for cleaning purposes. Why?
Ans: Soaps with sodium salts are made by heating fat (the glycerol ester of a fatty acid) with an aqueous sodium hydroxide solution. Soaps containing sodium and potassium are the only ones that are...
Birth control pills essentially contain a mixture of synthetic estrogen and progesterone. What are estrogen and progesterone? Why are they used in. birth control pills?
Ans: Estrogen and progesterone are female hormones that are linked to ovulation and menstruation in women. They also play a role in pregnancy. Birth control pills contain a mix of both hormones and...
The structure below is given as:
i. Identify the compound. Ans: The compound given in the structure is bithionol. It is an antiseptic ii. What is its use? Ans: Being an antiseptic, bithionol is used to cure wounds and treat minor...
Calculate the half-life of a first order reaction from their rate constants given below:
(a) 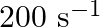
(b) 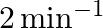
(c) 4 years 
Solution: (a) Half life, $t_{\frac{1}{2}}=\frac{0.693}{k}$ $$ \begin{array}{l} =\frac{0.693}{200 \mathrm{~s}^{-1}} \\ =3.47 \times 10^{-3} s \text { (Approximately) } \end{array} $$ (b)...
A reaction is first order in A and second order in B.
(i) Write the differential rate equation.
(ii) How is the rate affected on increasing the concentration of B three times?
(iii) How is the rate affected when the concentrations of both 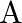 and
and 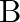 are doubled?
are doubled?
Solution: (a) The differential rate equation will be $$ \begin{array}{l} 5.07 \times 10^{-5}=k[0.20]^{x}[0.30]^{y} \\ 5.07 \times 10^{-5}=k[0.20]^{x}[0.10]^{y} \\ 1.43 \times...
In a pseudo-first-order reaction in water, the following results were obtained: \begin{tabular}{|l|l|l|l|l|} \hline 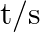 & 0 & 30 & 60 & 90 \\ \hline
& 0 & 30 & 60 & 90 \\ \hline![Rendered by QuickLaTeX.com [\mathrm{Ester}] \mathrm{mol} / \mathrm{L}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ca8d3c9d2794d5cbd8e09f4843bd5e9f_l3.png) &
& 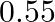 &
& 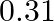 &
& 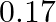 &
& 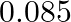 \\ \hline \end{tabular} Calculate the average rate of reaction between the time interval 30 to 60 seconds.
\\ \hline \end{tabular} Calculate the average rate of reaction between the time interval 30 to 60 seconds.
Solution: (a) Avg rate of reaction between the time intervals, 30 to 60 seconds, $$ \begin{array}{l} =\frac{\left.d E_{\text {ster }}\right]}{d t} \\ =\frac{0.31-0.17}{60-30} \\ =\frac{0.14}{30} \\...
What is the effect of temperature on the rate constant of a reaction? How can this effect of temperature on rate constant be represented quantitatively?
Solution: When a temperature of $10^{\text {a }}$ rises for a chemical reaction then the rate constant increases and becomes near to double of its original value. The temperature effect on the rate...
How do emulsifires stabilise emulsion? Name two emulsifiers.
Solution: By decreasing the interfacial tension between two liquids that form the emulsion, emulsifiers stabilize an emulsion. Long-chain molecules with polar groups are emulsifiers. Examples:...
What are emulsions? What are their different types? Give example of each type.
Solution: Emulsion is defined as the colloidal solution in which both the dispersed phase and dispersion medium are liquids. There are two types of emulsions: (a) Oil in water type: Here, water is...
Explain what is observed
(i) when a beam of light is passed through a colloidal sol. (ii) an electrolyte, $\mathrm{NaCl}$ is added to hydrated ferric oxide
How are colloids classified on the basis of
(i) physical states of components
(ii) nature of dispersed phase and
(iii) interaction between dispersed phase and dispersion medium?
Solution: Colloids can be classified on various basis: (i) By components we mean the dispersed phase and dispersion medium i.e. the physical state of the components. Therefore, we can have eight...
What are enzymes? Write in brief the mechanism of enzyme catalysis.
Solution: Protein molecules of high molecular masses are termed as enzymes. Colloidal solutions are formed when these are dissolved in water. These are complex, nitrogenous organic compounds...
What is the difference between multimolecular and macromolecular colloids? Give one example of each. How are associated colloids different from these two types of colloids?
Solution: (i) In multi-molecular colloids, the colloidal particles are an aggregate of atoms or small molecules with a diameter of less than $1 \mathrm{~nm}$. Van der Waal's forces of attraction are...
What are Iyophilic and lyophobic sols? Give one example of each type. Why are hydrophobic sols easily coagulated?
Solution: (i) Lyophilic sols: Lyophilic sols are colloidal sols that are formed by mixing substances such as gum, gelatin, starch, etc. with a suitable liquid (dispersion medium). These sols are...
Discuss the effect of pressure and temperature on the adsorption of gases on solids.
Solution: Effect of pressure: Adsorption increases with an increase in pressure, since adsorption is a reversible process and is accompanied by a decrease in pressure. Effect of temperature: In...
Define the term ‘point defeets’? Mention the main difference between stoichiometric and non-stoichiometric point defects.
Ans: Point defect - As the name implies, point faults occur exclusively at or around a single lattice point. They are not spatially expanded in any way. Stoichiometric defect do not affect the...
What is meant by non-stoichiometric defect? Ionic solids which have anionic vacancies due to metal excess defect develop color. Explain with the help of suitable example.
Ans: Non-stoichiometric flaws are those imperfections that do not affect the crystalline substance's stoichiometry. The anionic sites contain electrons, which cause the color to develop. As a result...
Explain how vacancies are introduced in a solid NaCl crystal when divalent cations are added to it,
Ans: A higher-valence cation is introduced as an impurity in an ionic solid. Less than 2 higher-valance ions replace two or more lower-valance cations or ions. Few locations are left empty in order...
Explain how much portion of an atom is located at
(a) corner and
Ans: In the corner of a cubic unit cell, eight neighboring unit cells share the same atom. One unit cell therefore shares $1 / 8$ th of the atom. (b) body centre Ans: Each unit cell in a cubic unit...
Derive the formula for the density of a crystal whose length of the edge of the unit cell is known?
Ans: Let the edge of the unit cell = a pm Number of atoms present per unit cell $=Z$ The atomic mass of the element is represented as $=\mathrm{M}$. So, the volume of the unit cell $=(a p...
Ans: This indicates that the arrangement of spheres in every fourth layer is the same as the arrangement of spheres in every first layer, and so on. Ultimately, this results in
What do you understand by the following types of stacking sequences: What kind of lattices do these sequences lead to? (a) AB AB ……
Ans: This indicates that the arrangement of spheres in every third layer is the same as that in the first. As a result, a hexagonal tight packing is obtained.
Why is graphite soft lubricant and good conductor of electricity?
Ans: A soft lubricant, graphite is an excellent conductor of electricity because its electrons are delocalized and free to flow about in the crystal structure. In addition to being an excellent...
Why is graphite soft lubricant and good conductor of electricity?
Ans: A soft lubricant, graphite is an excellent conductor of electricity because its electrons are delocalized and free to flow about in the crystal structure. In addition to being an excellent...
Window glass panes of old buildings are thicker at the bottom than at the top.
Ans: Glass is a supercooled liquid since it flows. Bottom glass panes are thicker than top glass panes.
